COVID-19 vaccine-induced Stevens-Johnson syndrome
- PMID: 34081806
- PMCID: PMC8239684
- DOI: 10.1111/ced.14784
COVID-19 vaccine-induced Stevens-Johnson syndrome
Abstract
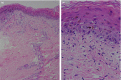
Herein, we are reporting a case of Stevens–Johnson syndrome due to COVID‐19 vaccine, which has been proved by history taking, clinical examination and histopathology. To the best of our knowledge we are reporting the first case of COVID‐19 vaccine‐induced Stevens–Johnson syndrome.
Figures

Comment in
-
Stevens-Johnson syndrome after ChAdOx1 nCoV-19 vaccine.Indian J Dermatol Venereol Leprol. 2022 Sep-Oct;88(5):702. doi: 10.25259/IJDVL_941_2021. Indian J Dermatol Venereol Leprol. 2022. PMID: 35986626 No abstract available.
References
-
- Ramasamy MN, Minassian AM, Ewer KJ et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet 2021; 396: 1979–93. (pu blished corrections appear in Lancet 2021; 396: 1978 and Lancet 2021; 397: 1350). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

