High-Fidelity Sequence-Selective Duplex Formation by Recognition-Encoded Melamine Oligomers
- PMID: 34081864
- PMCID: PMC8213060
- DOI: 10.1021/jacs.1c02275
High-Fidelity Sequence-Selective Duplex Formation by Recognition-Encoded Melamine Oligomers
Abstract
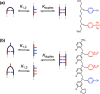
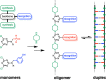
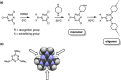
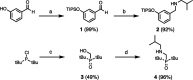
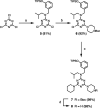
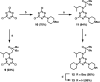
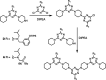
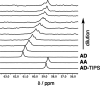
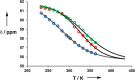
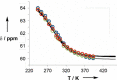
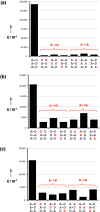
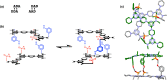
Melamine oligomers composed of repeating triazine-piperidine units and equipped with phenol and phosphine oxide side-chains form H-bonded duplexes. The melamine backbone provides sufficient rigidity to prevent intramolecular folding of oligomers up to three recognition units in length, leading to reliable duplex formation between sequence complementary oligomers. NMR spectroscopy and isothermal titration calorimetry (ITC) were used to characterize the self-assembly properties of the oligomers. For length-complementary homo-oligomers, duplex formation in toluene is characterized by an increase in stability of an order of magnitude for every base-pair added to the chain. NMR spectra of dilute solutions of the AD 2-mer show that intramolecular H-bonding between neighboring recognition units on the chain (1,2-folding) does not occur. NMR spectra of dilute solutions of both the AAD and the ADD 3-mer show that 1,3-folding does not take place either. ITC was used to characterize interactions between all pairwise combinations of the six different 3-mer sequences, and the sequence complementary duplexes are approximately an order of magnitude more stable than duplexes with a single base mismatch. High-fidelity duplex formation combined with the synthetic accessibility of the monomer building blocks makes these systems attractive targets for further investigation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Merrifield R. B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85 (14), 2149.10.1021/ja00897a025. - DOI
-
- Köster H. Polymer support oligonucleotide synthesis VI: Use of inorganic carriers. Tetrahedron Lett. 1972, 13, 1527.10.1016/S0040-4039(01)84677-3. - DOI
-
- Gough G. R.; Brunden M. J.; Gilham P. T. Recovery and recycling of synthetic units in the construction of oligodeoxyribonucleotides on solid supports. Tetrahedron Lett. 1981, 22, 4177.10.1016/S0040-4039(01)82097-9. - DOI
-
- Köster H.; Stumpe A.; Wolter A. Polymer support oligonucleotide synthesis 13: Rapid and efficient synthesis of oligodeoxynucleotides on porous glass support using triester approach. Tetrahedron Lett. 1983, 24, 747.10.1016/S0040-4039(00)81515-4. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

