SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment
- PMID: 34081912
- PMCID: PMC8130512
- DOI: 10.1016/j.cmet.2021.05.013
SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment
Abstract
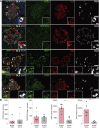
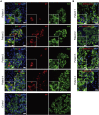
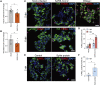
Emerging evidence points toward an intricate relationship between the pandemic of coronavirus disease 2019 (COVID-19) and diabetes. While preexisting diabetes is associated with severe COVID-19, it is unclear whether COVID-19 severity is a cause or consequence of diabetes. To mechanistically link COVID-19 to diabetes, we tested whether insulin-producing pancreatic β cells can be infected by SARS-CoV-2 and cause β cell depletion. We found that the SARS-CoV-2 receptor, ACE2, and related entry factors (TMPRSS2, NRP1, and TRFC) are expressed in β cells, with selectively high expression of NRP1. We discovered that SARS-CoV-2 infects human pancreatic β cells in patients who succumbed to COVID-19 and selectively infects human islet β cells in vitro. We demonstrated that SARS-CoV-2 infection attenuates pancreatic insulin levels and secretion and induces β cell apoptosis, each rescued by NRP1 inhibition. Phosphoproteomic pathway analysis of infected islets indicates apoptotic β cell signaling, similar to that observed in type 1 diabetes (T1D). In summary, our study shows SARS-CoV-2 can directly induce β cell killing.
Keywords: ACE2; COVID-19; SARS-CoV-2; SARS-CoV-2 spike protein; apoptosis; insulin; neuropilin 1; pancreatic beta cell; phosphoproteomics; type 1 diabetes.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
SARS-CoV-2 infection of islet β cells: Evidence and implications.Cell Rep Med. 2021 Aug 17;2(8):100380. doi: 10.1016/j.xcrm.2021.100380. Cell Rep Med. 2021. PMID: 34423322 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

