Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity
- PMID: 34081922
- PMCID: PMC8320529
- DOI: 10.1016/j.cell.2021.05.021
Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity
Abstract
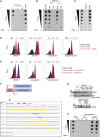
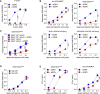
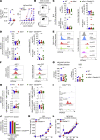
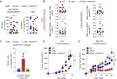
Cross-presentation of antigens from dead tumor cells by type 1 conventional dendritic cells (cDC1s) is thought to underlie priming of anti-cancer CD8+ T cells. cDC1 express high levels of DNGR-1 (a.k.a. CLEC9A), a receptor that binds to F-actin exposed by dead cell debris and promotes cross-presentation of associated antigens. Here, we show that secreted gelsolin (sGSN), an extracellular protein, decreases DNGR-1 binding to F-actin and cross-presentation of dead cell-associated antigens by cDC1s. Mice deficient in sGsn display increased DNGR-1-dependent resistance to transplantable tumors, especially ones expressing neoantigens associated with the actin cytoskeleton, and exhibit greater responsiveness to cancer immunotherapy. In human cancers, lower levels of intratumoral sGSN transcripts, as well as presence of mutations in proteins associated with the actin cytoskeleton, are associated with signatures of anti-cancer immunity and increased patient survival. Our results reveal a natural barrier to cross-presentation of cancer antigens that dampens anti-tumor CD8+ T cell responses.
Keywords: CLEC9A; DNGR-1; F-actin; cancer immunity; cross-presentation; dendritic cells; secreted gelsolin.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.G., O.S., K.H.J.L., N.S., O.G., S.Z., S.S., P.C., and C.R.S. are named as inventors on a patent application on the use of sGSN for immunotherapies. C.R.S. owns stock options and/or is a paid consultant for Bicara Therapeutics, Montis Biosciences, Oncurious NV, Bicycle Therapeutics, and Sosei Heptares. C.R.S. holds a professorship at Imperial College London and honorary professorships at University College London and King’s College London. None of these activities are related to this work.
Figures










References
-
- Ahrens S., Zelenay S., Sancho D., Hanč P., Kjær S., Feest C., Fletcher G., Durkin C., Postigo A., Skehel M. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity. 2012;36:635–645. - PubMed
-
- Asano K., Nabeyama A., Miyake Y., Qiu C.-H., Kurita A., Tomura M., Kanagawa O., Fujii S., Tanaka M. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. - PubMed
-
- Balam S., Kesselring R., Elke E., Blaimer S., Evert K., Evert M., Schlitt H.J., Geissler E.K., van Blijswijk J., Lee S. Cross-presentation of dead-cell-associated antigens by DNGR-1+ dendritic cells contributes to chronic allograft rejection in mice. Eur. J. Immunol. 2020;50:2041–2054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

