The amygdala modulates prepulse inhibition of the auditory startle reflex through excitatory inputs to the caudal pontine reticular nucleus
- PMID: 34082731
- PMCID: PMC8176709
- DOI: 10.1186/s12915-021-01050-z
The amygdala modulates prepulse inhibition of the auditory startle reflex through excitatory inputs to the caudal pontine reticular nucleus
Abstract
Background: Sensorimotor gating is a fundamental pre-attentive process that is defined as the inhibition of a motor response by a sensory event. Sensorimotor gating, commonly measured using the prepulse inhibition (PPI) of the auditory startle reflex task, is impaired in patients suffering from various neurological and psychiatric disorders. PPI deficits are a hallmark of schizophrenia, and they are often associated with attention and other cognitive impairments. Although the reversal of PPI deficits in animal models is widely used in pre-clinical research for antipsychotic drug screening, the neurotransmitter systems and synaptic mechanisms underlying PPI are still not resolved, even under physiological conditions. Recent evidence ruled out the longstanding hypothesis that PPI is mediated by midbrain cholinergic inputs to the caudal pontine reticular nucleus (PnC). Instead, glutamatergic, glycinergic, and GABAergic inhibitory mechanisms are now suggested to be crucial for PPI, at the PnC level. Since amygdalar dysfunctions alter PPI and are common to pathologies displaying sensorimotor gating deficits, the present study was designed to test that direct projections to the PnC originating from the amygdala contribute to PPI.
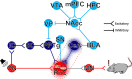
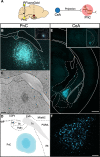
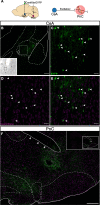
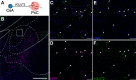
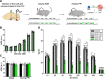
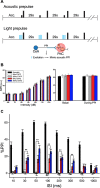
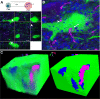
Results: Using wild type and transgenic mice expressing eGFP under the control of the glycine transporter type 2 promoter (GlyT2-eGFP mice), we first employed tract-tracing, morphological reconstructions, and immunohistochemical analyses to demonstrate that the central nucleus of the amygdala (CeA) sends glutamatergic inputs lateroventrally to PnC neurons, including GlyT2+ cells. Then, we showed the contribution of the CeA-PnC excitatory synapses to PPI in vivo by demonstrating that optogenetic inhibition of this connection decreases PPI, and optogenetic activation induces partial PPI. Finally, in GlyT2-Cre mice, whole-cell recordings of GlyT2+ PnC neurons in vitro paired with optogenetic stimulation of CeA fibers, as well as photo-inhibition of GlyT2+ PnC neurons in vivo, allowed us to implicate GlyT2+ neurons in the PPI pathway.
Conclusions: Our results uncover a feedforward inhibitory mechanism within the brainstem startle circuit by which amygdalar glutamatergic inputs and GlyT2+ PnC neurons contribute to PPI. We are providing new insights to the clinically relevant theoretical construct of PPI, which is disrupted in various neuropsychiatric and neurological diseases.
Keywords: Amygdala; Caudal pontine reticular nucleus; Electrophysiology; Optogenetics; Sensorimotor gating; Startle.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
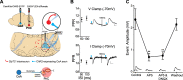


Similar articles
-
Deciphering the role of brainstem glycinergic neurons during startle and prepulse inhibition.Brain Res. 2024 Aug 1;1836:148938. doi: 10.1016/j.brainres.2024.148938. Epub 2024 Apr 13. Brain Res. 2024. PMID: 38615924
-
The Role of Cholinergic Midbrain Neurons in Startle and Prepulse Inhibition.J Neurosci. 2018 Oct 10;38(41):8798-8808. doi: 10.1523/JNEUROSCI.0984-18.2018. Epub 2018 Aug 31. J Neurosci. 2018. PMID: 30171090 Free PMC article.
-
Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit?J Neurosci. 1994 Mar;14(3 Pt 1):1176-94. doi: 10.1523/JNEUROSCI.14-03-01176.1994. J Neurosci. 1994. PMID: 8120618 Free PMC article.
-
Brain stem circuits mediating prepulse inhibition of the startle reflex.Psychopharmacology (Berl). 2001 Jul;156(2-3):216-24. doi: 10.1007/s002130100794. Psychopharmacology (Berl). 2001. PMID: 11549224 Review.
-
Prepulse inhibition of the acoustic startle reflex and P50 gating in aging and alzheimer's disease.Ageing Res Rev. 2020 May;59:101028. doi: 10.1016/j.arr.2020.101028. Epub 2020 Feb 21. Ageing Res Rev. 2020. PMID: 32092463 Review.
Cited by
-
A Computational Model for the Simulation of Prepulse Inhibition and Its Modulation by Cortical and Subcortical Units.Brain Sci. 2024 May 15;14(5):502. doi: 10.3390/brainsci14050502. Brain Sci. 2024. PMID: 38790479 Free PMC article.
-
Neurodegeneration Within the Amygdala Is Differentially Induced by Opioid and HIV-1 Tat Exposure.Front Neurosci. 2022 May 4;16:804774. doi: 10.3389/fnins.2022.804774. eCollection 2022. Front Neurosci. 2022. PMID: 35600626 Free PMC article.
-
PDE4B Missense Variant Increases Susceptibility to Post-traumatic Stress Disorder-Relevant Phenotypes in Mice.J Neurosci. 2024 Oct 23;44(43):e0137242024. doi: 10.1523/JNEUROSCI.0137-24.2024. J Neurosci. 2024. PMID: 39256048 Free PMC article.
-
The Startle Response and Prepulse Inhibition in Psychosis and Violence: A Combined Electromyography and Electroencephalography Study.Schizophr Bull Open. 2024 Dec 28;6(1):sgae031. doi: 10.1093/schizbullopen/sgae031. eCollection 2025 Jan. Schizophr Bull Open. 2024. PMID: 39882404 Free PMC article.
-
Peripubertal antagonism of corticotropin-releasing factor receptor 1 results in sustained changes in behavioral plasticity and the transcriptomic profile of the amygdala.bioRxiv [Preprint]. 2025 Jan 4:2024.08.14.607957. doi: 10.1101/2024.08.14.607957. bioRxiv. 2025. Update in: Neuroscience. 2025 Feb 16;567:261-270. doi: 10.1016/j.neuroscience.2025.01.007. PMID: 39185241 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

