Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates
- PMID: 34082772
- PMCID: PMC8173980
- DOI: 10.1186/s12974-021-02158-3
Crosstalk between astrocytes and microglia results in increased degradation of α-synuclein and amyloid-β aggregates
Abstract
Background: Alzheimer's disease (AD) and Parkinson's disease (PD) are characterized by brain accumulation of aggregated amyloid-beta (Aβ) and alpha-synuclein (αSYN), respectively. In order to develop effective therapies, it is crucial to understand how the Aβ/αSYN aggregates can be cleared. Compelling data indicate that neuroinflammatory cells, including astrocytes and microglia, play a central role in the pathogenesis of AD and PD. However, how the interplay between the two cell types affects their clearing capacity and consequently the disease progression remains unclear.
Methods: The aim of the present study was to investigate in which way glial crosstalk influences αSYN and Aβ pathology, focusing on accumulation and degradation. For this purpose, human-induced pluripotent cell (hiPSC)-derived astrocytes and microglia were exposed to sonicated fibrils of αSYN or Aβ and analyzed over time. The capacity of the two cell types to clear extracellular and intracellular protein aggregates when either cultured separately or in co-culture was studied using immunocytochemistry and ELISA. Moreover, the capacity of cells to interact with and process protein aggregates was tracked using time-lapse microscopy and a customized "close-culture" chamber, in which the apical surfaces of astrocyte and microglia monocultures were separated by a <1 mm space.
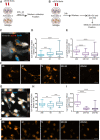
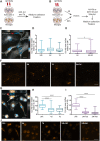
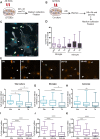
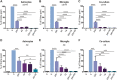
Results: Our data show that intracellular deposits of αSYN and Aβ are significantly reduced in co-cultures of astrocytes and microglia, compared to monocultures of either cell type. Analysis of conditioned medium and imaging data from the "close-culture" chamber experiments indicate that astrocytes secrete a high proportion of their internalized protein aggregates, while microglia do not. Moreover, co-cultured astrocytes and microglia are in constant contact with each other via tunneling nanotubes and other membrane structures. Notably, our live cell imaging data demonstrate that microglia, when attached to the cell membrane of an astrocyte, can attract and clear intracellular protein deposits from the astrocyte.
Conclusions: Taken together, our data demonstrate the importance of astrocyte and microglia interactions in Aβ/αSYN clearance, highlighting the relevance of glial cellular crosstalk in the progression of AD- and PD-related brain pathology.
Keywords: Alzheimer’s disease; Amyloid-β; Astrocyte; Co-culture; Crosstalk; Degradation; Microglia; Parkinson’s disease; Tunneling nanotube; α-Synuclein.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

