A triple-blinded crossover study to evaluate the short-term safety of sweet manioc starch for the treatment of glycogen storage disease type Ia
- PMID: 34082801
- PMCID: PMC8173866
- DOI: 10.1186/s13023-021-01877-3
A triple-blinded crossover study to evaluate the short-term safety of sweet manioc starch for the treatment of glycogen storage disease type Ia
Abstract
Background: Glycogen storage disease type 1a (GSD Ia) is characterized by severe fasting hypoglycemia. The clinical management includes the administration of uncooked cornstarch (UCCS). Although such a diet approach is effective in achieving euglycemia, its impact on the quality of life of patients should be considered. In vitro analyses suggest a longer release of glucose when using sweet manioc starch (SMS).
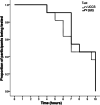
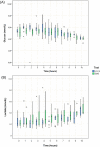
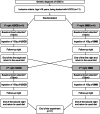
Methods: We compared the efficacy and safety of the administration of SMS and UCCS during a short-fasting challenge in patients with GSD Ia in a randomized, triple-blind, phase I/II, cross-over study. GSD Ia patients aged ≥ 16 years and treated with UCCS were enrolled. Participants were hospitalized for two consecutive nights, receiving UCCS or SMS in each night. After the administration of the starches, glucose, lactate and insulin levels were measured in 1-h interval throughout the hospitalization period. The procedures were interrupted after 10 h of fasting or in a hypoglycemic episode (< 3.88 mmol/L).
Results: Eleven individuals (mean age: 21.6 ± 4.3 years; all presenting body mass index > 25 kg/m2) participated in the study. The average fasting period was 8.2 ± 2.0 h for SMS and 7.7 ± 2.3 h for UCCS (p = 0.04). SMS maintained euglycemia for a greater period over UCCS. Increased lactate concentrations were detected even in absence of hypoglycemia, not being influenced by the different starches investigated (p = 0.17). No significant difference was found in total cholesterol, HDL, triglycerides and uric acid levels in both arms. None of the patients showed severe adverse events.
Conclusions: SMS appears to be non-inferior to UCCS in the maintenance of euglycemia, thus emerging as a promising alternative to the treatment of GSD Ia.
Keywords: Cornstarch; Dietary treatment; Hepatic glycogen storage disease; Inborn errors of metabolism; Sweet manioc starch; Treatment strategies.
Conflict of interest statement
TN is currently an employee of Ultragenyx Farmacêutica Brasil LTDA and hold stock in Ultragenyx Pharmaceutical Inc. All other authors declare no conflicts of interest.
Figures
Similar articles
-
Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs.Nutrients. 2021 Oct 27;13(11):3828. doi: 10.3390/nu13113828. Nutrients. 2021. PMID: 34836082 Free PMC article. Review.
-
Potential use of other starch sources in the treatment of glycogen storage disease type Ia - an in vitro study.Orphanet J Rare Dis. 2024 Jul 30;19(1):283. doi: 10.1186/s13023-024-03201-1. Orphanet J Rare Dis. 2024. PMID: 39080776 Free PMC article.
-
Impact of hematopoietic stem cell transplantation in glycogen storage disease type Ib: A single-subject research design using 13C-glucose breath test.Mol Genet Metab Rep. 2023 Jan 3;34:100955. doi: 10.1016/j.ymgmr.2023.100955. eCollection 2023 Mar. Mol Genet Metab Rep. 2023. PMID: 36632325 Free PMC article.
-
Sleep and quality of life of patients with glycogen storage disease on standard and modified uncooked cornstarch.Mol Genet Metab. 2018 Mar;123(3):326-330. doi: 10.1016/j.ymgme.2017.09.003. Epub 2017 Sep 11. Mol Genet Metab. 2018. PMID: 29223626
-
Clinical features of gout in adult patients with type Ia glycogen storage disease: a single-centre retrospective study and a review of literature.Arthritis Res Ther. 2022 Feb 26;24(1):58. doi: 10.1186/s13075-021-02706-5. Arthritis Res Ther. 2022. PMID: 35219330 Free PMC article. Review.
Cited by
-
Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs.Nutrients. 2021 Oct 27;13(11):3828. doi: 10.3390/nu13113828. Nutrients. 2021. PMID: 34836082 Free PMC article. Review.
-
Feeding Difficulties in Children with Hepatic Glycogen Storage Diseases Identified by a Brazilian Portuguese Validated Screening Tool.Nutrients. 2025 May 23;17(11):1758. doi: 10.3390/nu17111758. Nutrients. 2025. PMID: 40507027 Free PMC article.
-
Potential use of other starch sources in the treatment of glycogen storage disease type Ia - an in vitro study.Orphanet J Rare Dis. 2024 Jul 30;19(1):283. doi: 10.1186/s13023-024-03201-1. Orphanet J Rare Dis. 2024. PMID: 39080776 Free PMC article.
-
A case of glycogen storage disease type Ⅰa with gout as the first manifestation.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023 Apr 25;52(2):230-236. doi: 10.3724/zdxbyxb-2022-0530. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 37283108 Free PMC article. Chinese, English.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

