Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) moonlights as an adhesin in Mycoplasma hyorhinis adhesion to epithelial cells as well as a plasminogen receptor mediating extracellular matrix degradation
- PMID: 34082810
- PMCID: PMC8173509
- DOI: 10.1186/s13567-021-00952-8
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) moonlights as an adhesin in Mycoplasma hyorhinis adhesion to epithelial cells as well as a plasminogen receptor mediating extracellular matrix degradation
Abstract
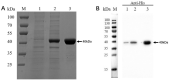
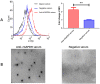
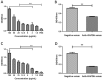
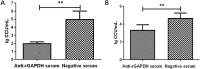
Mycoplasma hyorhinis infects pigs causing polyserositis and polyarthritis, and has also been reported in a variety of human tumor tissues. The occurrence of disease is often linked with the systemic invasion of the pathogen. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH), one of the key enzymes of glycolysis, was reported as a surface multifunctional molecule in several bacteria. Here, we investigated whether GAPDH could manifest binary functions; as an adhesin to promote colonization as well as a plasminogen receptor functioning in extracellular matrix (ECM) degradation to promote systemic invasion. The surface localization of GAPDH was observed in M. hyorhinis with flow cytometry and colony blot analysis. Recombinant GAPDH (rGAPDH) was found to be able to bind porcine-derived PK-15 and human-derived NCI-H292 cells. The incubation with anti-GAPDH antibody significantly decreased the adherence of M. hyorhinis to both cell lines. To investigate its function in recruiting plasminogen, firstly, the interaction between rGAPDH and plasminogen was demonstrated by ELISA and Far-Western blot assay. The activation of the rGAPDH-bound plasminogen into plasmin was proved by using a chromogenic substrate, and furtherly confirmed to degrade extracellular matrix by using a reconstituted ECM. Finally, the ability of rGAPDH to bind different ECM components was demonstrated, including fibronectin, laminin, collagen type IV and vitronectin. Collectively, our data imply GAPDH as an important adhesion factor of M. hyrohinis and a receptor for hijacking host plasminogen to degrade ECM. The multifunction of GAPDH to bind both plasminogen and ECM components is believed to increase the targeting of proteolysis and facilitate the dissemination of M. hyorhinis.
Keywords: Adhesion; Extracellular matrix; GAPDH; M. hyorhinis; Plasminogen.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Hijacking of Host Plasminogen by Mesomycoplasma (Mycoplasma) hyopneumoniae via GAPDH: an Important Virulence Mechanism To Promote Adhesion and Extracellular Matrix Degradation.Microbiol Spectr. 2023 Jun 15;11(3):e0021823. doi: 10.1128/spectrum.00218-23. Epub 2023 May 18. Microbiol Spectr. 2023. PMID: 37199643 Free PMC article.
-
A multifunctional enolase mediates cytoadhesion and interaction with host plasminogen and fibronectin in Mycoplasma hyorhinis.Vet Res. 2022 Mar 25;53(1):26. doi: 10.1186/s13567-022-01041-0. Vet Res. 2022. PMID: 35337383 Free PMC article.
-
Mycobacterium tuberculosis glyceraldehyde-3-phosphate dehydrogenase plays a dual role-As an adhesin and as a receptor for plasmin(ogen).Cell Microbiol. 2021 May;23(5):e13311. doi: 10.1111/cmi.13311. Epub 2021 Feb 16. Cell Microbiol. 2021. PMID: 33486886
-
[Mechanisms of glyceraldehyde 3-phosphosphate dehydrogenaseis in bacteria adhesion - A review].Wei Sheng Wu Xue Bao. 2016 Sep;56(9):1398-1405. Wei Sheng Wu Xue Bao. 2016. PMID: 29738212 Review. Chinese.
-
The cell surface adhesins of Mycobacterium tuberculosis.Microbiol Res. 2020 Feb;232:126392. doi: 10.1016/j.micres.2019.126392. Epub 2019 Dec 9. Microbiol Res. 2020. PMID: 31841935 Review.
Cited by
-
Characterization of Mutations in DNA Gyrase and Topoisomerase IV in Field Strains and In Vitro Selected Quinolone-Resistant Mycoplasma hyorhinis Mutants.Antibiotics (Basel). 2022 Apr 7;11(4):494. doi: 10.3390/antibiotics11040494. Antibiotics (Basel). 2022. PMID: 35453245 Free PMC article.
-
The Mycoplasma spp. 'Releasome': A New Concept for a Long-Known Phenomenon.Front Microbiol. 2022 Apr 15;13:853440. doi: 10.3389/fmicb.2022.853440. eCollection 2022. Front Microbiol. 2022. PMID: 35495700 Free PMC article. Review.
-
Interactions of Mycoplasma hyopneumoniae and/or Mycoplasma hyorhinis with Streptococcus suis Serotype 2 Using In Vitro Co-Infection Models with Swine Cells.Pathogens. 2023 Jun 22;12(7):866. doi: 10.3390/pathogens12070866. Pathogens. 2023. PMID: 37513713 Free PMC article.
-
Mycoplasma synoviae elongation factor thermo stable is an adhesion-associated protein that enters cells by endocytosis and stimulates DF-1 cell proliferation.BMC Vet Res. 2024 Nov 19;20(1):522. doi: 10.1186/s12917-024-04374-4. BMC Vet Res. 2024. PMID: 39558348 Free PMC article.
-
FabG moonlights as an extracellular adhesin mediates cytoadhesion of Streptococcus suis via interaction with plasminogen.BMC Microbiol. 2025 Jul 4;25(1):412. doi: 10.1186/s12866-025-04129-7. BMC Microbiol. 2025. PMID: 40615777 Free PMC article.
References
-
- Pieters M, Maes D (2019) Mycoplasmosis. In: Zimmermann JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, Zhang J Diseases of swine (11 ed). Hoboken, NJ, Wiley. pp: 863–883
-
- Kim MK, Shin SJ, Lee HM, Choi HS, Jeong J, Kim H, Paik SS, Kim M, Choi D, Ryu CJ. Mycoplasma infection promotes tumor progression via interaction of the mycoplasmal protein p37 and epithelial cell adhesion molecule in hepatocellular carcinoma. Cancer Lett. 2019;454:44–52. doi: 10.1016/j.canlet.2019.04.007. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 31770193/National Natural Science Foundation of China
- 31700158/National Natural Science Foundation of China
- BRA2020370/333 High-level Personnel Training Project of Jiangsu Province of China
- NY-015/Six Talent Peaks Project in Jiangsu Province
- CX(20)3090/Jiangsu Agricultural Science and Technology Independent Innovation Fund
LinkOut - more resources
Full Text Sources
Research Materials

