B cell subset composition segments clinically and serologically distinct groups in chronic cutaneous lupus erythematosus
- PMID: 34083207
- PMCID: PMC8906255
- DOI: 10.1136/annrheumdis-2021-220349
B cell subset composition segments clinically and serologically distinct groups in chronic cutaneous lupus erythematosus
Erratum in
-
Correction: B cell subset composition segments clinically and serologically distinct groups in chronic cutaneous lupus erythematosus.Ann Rheum Dis. 2022 Aug;81(8):e156. doi: 10.1136/annrheumdis-2021-220349corr1. Ann Rheum Dis. 2022. PMID: 35820697 No abstract available.
Abstract
Objective: While the contribution of B-cells to SLE is well established, its role in chronic cutaneous lupus erythematosus (CCLE) remains unclear. Here, we compare B-cell and serum auto-antibody profiles between patients with systemic lupus erythematosus (SLE), CCLE, and overlap conditions.
Methods: B-cells were compared by flow cytometry amongst healthy controls, CCLE without systemic lupus (CCLE+/SLE-) and SLE patients with (SLE+/CCLE+) or without CCLE (SLE+/CCLE-). Serum was analyed for autoreactive 9G4+, anti-double-stranded DNA, anti-chromatin and anti-RNA antibodies by ELISA and for anti-RNA binding proteins (RBP) by luciferase immunoprecipitation.
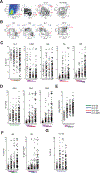
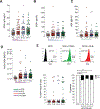
Results: Patients with CCLE+/SLE- share B-cell abnormalities with SLE including decreased unswitched memory and increased effector B-cells albeit at a lower level than SLE patients. Similarly, both SLE and CCLE+/SLE- patients have elevated 9G4+ IgG autoantibodies despite lower levels of anti-nucleic acid and anti-RBP antibodies in CCLE+/SLE-. CCLE+/SLE- patients could be stratified into those with SLE-like B-cell profiles and a separate group with normal B-cell profiles. The former group was more serologically active and more likely to have disseminated skin lesions.
Conclusion: CCLE displays perturbations in B-cell homeostasis and partial B-cell tolerance breakdown. Our study demonstrates that this entity is immunologically heterogeneous and includes a disease segment whose B-cell compartment resembles SLE and is clinically associated with enhanced serological activity and more extensive skin disease. This picture suggests that SLE-like B-cell changes in primary CCLE may help identify patients at risk for subsequent development of SLE. B-cell profiling in CCLE might also indentify candidates who would benefit from B-cell targeted therapies.
Keywords: B-lymphocytes; autoantibodies; autoimmunity; lupus erythematosus; systemic.
© Author(s) (or their employer(s)) 2021. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Yazdany JDE M Definition and Classification of Lupus and Lupus-Related Disorders. In: Wallace DHB, ed. Dubois’ Lupus Erythematosus. 9th ed. Philadelphia: Elsevier, 2018: 15–22.
-
- Rothfield N, Sontheimer RD, Bernstein M. Lupus erythematosus: systemic and cutaneous manifestations. Clin Dermatol 2006;24:348–62. - PubMed
-
- Werth VP. Clinical manifestations of cutaneous lupus erythematosus. Autoimmun Rev 2005;4:296–302. - PubMed
-
- Chong BF. Understanding how cutaneous lupus erythematosus progresses to systemic lupus erythematosus. JAMA Dermatol 2014;150:296. - PubMed
-
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol 2011;164:1335–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

