Aging and CNS Myeloid Cell Depletion Attenuate Breast Cancer Brain Metastasis
- PMID: 34083229
- PMCID: PMC9974011
- DOI: 10.1158/1078-0432.CCR-21-1549
Aging and CNS Myeloid Cell Depletion Attenuate Breast Cancer Brain Metastasis
Abstract
Purpose: Breast cancer diagnosed in young patients is often aggressive. Because primary breast tumors from young and older patients have similar mutational patterns, we hypothesized that the young host microenvironment promotes more aggressive metastatic disease.
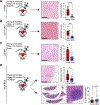
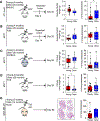
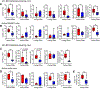
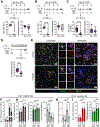
Experimental design: Triple-negative or luminal B breast cancer cell lines were injected into young and older mice side-by-side to quantify lung, liver, and brain metastases. Young and older mouse brains, metastatic and naïve, were analyzed by flow cytometry. Immune populations were depleted using antibodies or a colony-stimulating factor-1 receptor (CSF-1R) inhibitor, and brain metastasis assays were conducted. Effects on myeloid populations, astrogliosis, and the neuroinflammatory response were determined.
Results: Brain metastases were 2- to 4-fold higher in young as compared with older mouse hosts in four models of triple-negative or luminal B breast cancer; no age effect was observed on liver or lung metastases. Aged brains, naïve or metastatic, contained fewer resident CNS myeloid cells. Use of a CSF-1R inhibitor to deplete myeloid cells, including both microglia and infiltrating macrophages, preferentially reduced brain metastasis burden in young mice. Downstream effects of CSF-1R inhibition in young mice resembled that of an aged brain in terms of myeloid numbers, induction of astrogliosis, and Semaphorin 3A secretion within the neuroinflammatory response.
Conclusions: Host microenvironmental factors contribute to the aggressiveness of triple-negative and luminal B breast cancer brain metastasis. CSF-1R inhibitors may hold promise for young brain metastasis patients.
©2021 American Association for Cancer Research.
Figures

References
-
- Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 2008;26:3324–30. - PubMed
-
- Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol 2016;34:3308–14. - PubMed
-
- Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol 2010;21:1974–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

