First-in-Human Study of PF-06647020 (Cofetuzumab Pelidotin), an Antibody-Drug Conjugate Targeting Protein Tyrosine Kinase 7, in Advanced Solid Tumors
- PMID: 34083232
- PMCID: PMC9401513
- DOI: 10.1158/1078-0432.CCR-20-3757
First-in-Human Study of PF-06647020 (Cofetuzumab Pelidotin), an Antibody-Drug Conjugate Targeting Protein Tyrosine Kinase 7, in Advanced Solid Tumors
Abstract
Purpose: We investigated safety, tolerability, pharmacokinetics, and antitumor activity of the protein tyrosine kinase 7 (PTK7)-targeted, auristatin-based antibody-drug conjugate (ADC) PF-06647020/cofetuzumab pelidotin (NCT02222922).
Patients and methods: Patients received PF-06647020 intravenously every 3 weeks at 0.2-3.7 mg/kg or every 2 weeks at 2.1-3.2 mg/kg, in sequential dose escalation, following a modified toxicity probability interval method. In dose expansion, pretreated patients with advanced, platinum-resistant ovarian cancer, non-small cell lung cancer (NSCLC), or triple-negative breast cancer (TNBC) received PF-06647020 2.8 mg/kg every 3 weeks.
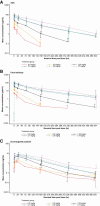
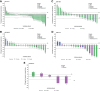
Results: The most common, treatment-related adverse events for PF-06647020 administered every 3 weeks were nausea, alopecia, fatigue, headache, neutropenia, and vomiting (45%-25%); 25% of patients had grade ≥ 3 neutropenia. Two patients experienced dose-limiting toxicities (grade 3 headache and fatigue) at the highest every 3 weeks dose evaluated. The recommended phase II dose was 2.8 mg/kg every 3 weeks. The overall safety profile observed with PF-06647020 administered every 2 weeks was similar to that of the every 3 weeks regimen. Systemic exposure for the ADC and total antibody generally increased in a dose-proportional manner. Antitumor activity was observed in treated patients with overall objective response rates of 27% in ovarian cancer (n = 63), 19% in NSCLC (n = 31), and 21% in TNBC (n = 29). Responders tended to have moderate or high PTK7 tumor expression by IHC.
Conclusions: This PTK7-targeted ADC demonstrated therapeutic activity in previously treated patients with ovarian cancer, NSCLC, and TNBC at a dose range of 2.1-3.2 mg/kg, supporting further clinical evaluation to refine dose, schedule, and predictive tissue biomarker testing in patients with advanced malignancies.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

