Azithromycin and hydroxychloroquine in hospitalised patients with confirmed COVID-19: a randomised double-blinded placebo-controlled trial
- PMID: 34083403
- PMCID: PMC8186006
- DOI: 10.1183/13993003.00752-2021
Azithromycin and hydroxychloroquine in hospitalised patients with confirmed COVID-19: a randomised double-blinded placebo-controlled trial
Abstract
Background: Combining the antibiotic azithromycin and hydroxychloroquine induces airway immunomodulatory effects, with the latter also having in vitro antiviral properties. This may improve outcomes in patients hospitalised for coronavirus disease 2019 (COVID-19).
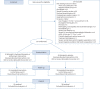
Methods: Placebo-controlled double-blind randomised multicentre trial. Patients aged ≥18 years, admitted to hospital for ≤48 h (not intensive care) with a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription PCR test were recruited. The intervention was 500 mg daily azithromycin for 3 days followed by 250 mg daily azithromycin for 12 days combined with 200 mg twice-daily hydroxychloroquine for all 15 days. The control group received placebo/placebo. The primary outcome was days alive and discharged from hospital within 14 days (DAOH14).
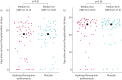
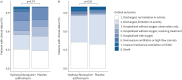
Results: After randomisation of 117 patients, at the first planned interim analysis, the data and safety monitoring board recommended stopping enrolment due to futility, based on pre-specified criteria. Consequently, the trial was terminated on 1 February 2021. 61 patients received the combined intervention and 56 patients received placebo. In the intervention group, patients had a median (interquartile range) 9.0 (3-11) DAOH14 versus 9.0 (7-10) DAOH14 in the placebo group (p=0.90). The primary safety outcome, death from all causes on day 30, occurred for one patient in the intervention group versus two patients receiving placebo (p=0.52), and readmittance or death within 30 days occurred for nine patients in the intervention group versus six patients receiving placebo (p=0.57).
Conclusions: The combination of azithromycin and hydroxychloroquine did not improve survival or length of hospitalisation in patients with COVID-19.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: P. Sivapalan reports fees from Boehringer Ingelheim, outside the submitted work. C.S. Ulrik reports fees from Boehringer Ingelheim, AZ, GSK, TEVA, Novartis, ALK-Abello, Mundipharma, Sanofi Genzyme, Orion Pharma and Actelion, outside the submitted work. K.E.J. Håkansson reports personal fees from AstraZeneca, Chiesi and TEVA, outside the submitted work. T. Biering-Sørensen has received research grants from GE Healthcare and Sanofi Pasteur, as well as personal fees from Sanofi Pasteur, Novartis and Amgen, outside the submitted work. None of the other authors have any conflicts of interest.
Figures
Comment in
-
Lessons learnt from hydroxychloroquine/azithromycin in treatment of COVID-19.Eur Respir J. 2022 Jan 6;59(1):2102002. doi: 10.1183/13993003.02002-2021. Print 2022 Jan. Eur Respir J. 2022. PMID: 34326192 Free PMC article.
References
-
- Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol 2011; 7: 718–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
