Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial
- PMID: 34083421
- PMCID: PMC8183204
- DOI: 10.1136/jitc-2021-002568
Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial
Erratum in
-
Correction: Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial.J Immunother Cancer. 2021 Aug;9(8):e002568corr1. doi: 10.1136/jitc-2021-002568corr1. J Immunother Cancer. 2021. PMID: 34376555 Free PMC article. No abstract available.
Abstract
Background: Head and neck squamous cell carcinomas (HNSCCs) are common malignancies caused by carcinogens, including tobacco and alcohol, or infection with human papillomavirus (HPV). Immune checkpoint inhibitors targeting the programmed cell death 1 (PD-1) pathway are effective against unresectable recurrent/metastatic HNSCC. Here, we explored the safety and efficacy of anti-PD-1 therapy in at-risk resectable HPV-positive and HPV-negative HNSCC in the neoadjuvant setting.
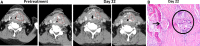
Methods: The phase I/II CheckMate 358 trial in virus-associated cancers assessed neoadjuvant nivolumab in patients with previously untreated, resectable HPV-positive or HPV-negative HNSCC. Patients received nivolumab 240 mg intravenously on days 1 and 15, with surgery planned by day 29. Safety/tolerability (primary endpoint) was assessed by monitoring adverse events (AEs) and surgical delays. Radiographic response was measured before surgery using RECIST v1.1, adapted for a single post-nivolumab evaluation. Pathologic specimens were examined for treatment response using immune-based criteria.
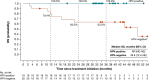
Results: From November 2015 to December 2017, 52 patients with AJCC (seventh edition) stage III-IV resectable HNSCC received neoadjuvant nivolumab (26 HPV-positive, 26 HPV-negative). Any-grade treatment-related AEs (TRAEs) occurred in 19 patients (73.1%) and 14 patients (53.8%) in the HPV-positive and HPV-negative cohorts, respectively; grade 3-4 TRAEs occurred in five (19.2%) and three patients (11.5%), respectively. No patient had a protocol-defined TRAE-related surgical delay (>4 weeks). Thirty-eight patients were reported as undergoing complete surgical resection, 10 had a planned post-nivolumab biopsy instead of definitive surgery due to a protocol misinterpretation, and four did not undergo surgery or biopsy, including two with tumor progression. Radiographic response rates in 49 evaluable patients were 12.0% and 8.3% in the HPV-positive and HPV-negative cohorts, respectively. There were no complete pathologic responses by site or central review in operated patients. Among 17 centrally evaluable HPV-positive tumors, one (5.9%) achieved major pathological response and three (17.6%) achieved partial pathologic response (pPR); among 17 centrally evaluable HPV-negative tumors, one (5.9%) achieved pPR.
Conclusions: Neoadjuvant nivolumab was generally safe and induced pathologic regressions in HPV-positive (23.5%) and HPV-negative (5.9%) tumors. Combinatorial neoadjuvant treatment regimens, and continued postoperative therapy for high-risk tumors, are warranted in future trials to enhance the efficacy of this approach.
Trial registration number: ClinicalTrials.gov NCT02488759; https://clinicaltrials.gov/ct2/show/NCT02488759.
Keywords: clinical trials as topic; head and neck neoplasms; immunotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RLF reports consulting or advisory from Bristol Myers Squibb (BMS), MedImmune, Merck, Lilly, Pfizer, Amgen, EMD Serono, PPD, Bain Capital Life Sciences, GlaxoSmithKline, Iovance Biotherapeutics, Numab Therapeutics AG, Oncorus, Ono Pharmaceutical, Regeneron, Novasenta, Aduro Biotech, MacroGenics, Nanobiotix, Torque Therapeutics, Lifescience Dynamics, Sanofi, and Zymeworks, Inc; and research funding from BMS, MedImmune, Merck, Tesaro, Novasenta, VentiRx, and AstraZeneca/MedImmune. WCS reports consulting from BMS and Regeneron. RL reports personal and institutional research funding from BMS. AG has nothing to disclose. UMM reports consulting and advisory from MSD Oncology, Roche, BMS, and Celgene; and travel accommodations from BMS, Celgene, Amgen, and Pierre Fabre. CK has nothing to disclose. WS reports honoraria from BMS and Array BioPharma; consulting or advisory from BMS, Novartis, Regeneron, ION Pharma, and Merck; research funding from Novartis, Merck, and Genentech; and institutional research funding from BMS. CHC reports consulting or advisory fees from BMS, CUE Biopharma, Ignyta, Mirati Therapeutics, and Sanofi; research funding from BMS, Ignyta, Lilly, Regeneron, IRX Therapeutics, and Lion Biotechnologies; and travel accommodation expenses from Mirati Therapeutics. LAD reports institutional expert input forum payments from MSD BV Netherlands; and institutional speaker fee payment from BMS. HG has nothing to disclose. SIC has nothing to disclose. LV has nothing to disclose. JMT reports consulting and advisory from BMS, MedImmune, Merck, Compugen, and Akoya Biosciences, and stock options from Akoya Biosciences. JES has nothing to disclose. JL reports employment and stock ownership from BMS. BL reports employment and stock ownership from BMS. TC reports employment and stock ownership from BMS. AB reports employment and stock ownership from BMS. SLT reports consulting or advisory from Five Prime Therapeutics, Immunocore, and Merck; travel accommodations from Five Prime Therapeutics, Merck, and BMS; research funding from BMS; stock ownership by her spouse in Tizona Therapeutics, DNAtrix, RAPT, WindMIL, Dragonfly Therapeutics, Ervaxx, Trieza Therapeutics, and Dracen Pharmaceuticals; consulting or advisory by her spouse in DNAtrix, RAPT, WindMIL, Dragonfly Therapeutics, Ervaxx, Amgen, AstraZeneca, Immunomic Therapeutics, Janssen Oncology, and Dynavax Technologies; royalties by her spouse in WindMIL, Immunomic Therapeutics, Arbor Pharmaceuticals, BMS, and NexImmune; and research funding by her spouse from Compugen.
Figures


References
-
- Blot WJ, McLaughlin JK, Winn DM, et al. . Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282–7. - PubMed
-
- Hashibe M, Brennan P, Benhamou S, et al. . Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst 2007;99:777–89. 10.1093/jnci/djk179 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
