Pre-emptive pharmacological inhibition of fatty acid-binding protein 4 attenuates kidney fibrosis by reprogramming tubular lipid metabolism
- PMID: 34083513
- PMCID: PMC8175732
- DOI: 10.1038/s41419-021-03850-1
Pre-emptive pharmacological inhibition of fatty acid-binding protein 4 attenuates kidney fibrosis by reprogramming tubular lipid metabolism
Abstract
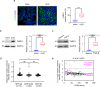
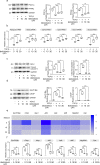
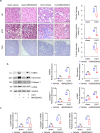
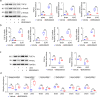
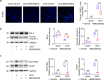
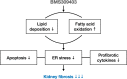
Kidney fibrosis is a hallmark of chronic kidney disease (CKD) progression that is caused by tubular injury and dysregulated lipid metabolism. Genetic abolition fatty acid-binding protein 4 (FABP4), a key lipid transporter, has been reported to suppress kidney interstitial fibrosis. However, the role and underlying mechanism of chemical inhibition of FABP4 in fibrotic kidney have not been well-documented. Here, we examined preemptive the effect of a FABP4 inhibitor, BMS309403, on lipid metabolism of tubular epithelial cells (TECs) and progression of kidney fibrosis. The expression of FABP4 was significantly elevated, concomitated with the accumulation of lipid droplets in TECs during kidney fibrosis. Treatment with BMS309403 alleviated lipid deposition of TECs, as well as interstitial fibrotic responses both in unilateral ureteral obstruction (UUO)-engaged mice and TGF-β-induced TECs. Moreover, BMS309403 administration enhanced fatty acid oxidation (FAO) in TECs by regulating peroxisome proliferator-activated receptor γ (PPARγ) and restoring FAO-related enzyme activities; In addition, BMS309403 markedly reduced cell lipotoxicity, such as endoplasmic reticulum (ER) stress and apoptosis in fibrotic kidney. Taken together, our results suggest that preemptive pharmacological inhibition of FABP4 by BMS309403 rebalances abnormal lipid metabolism in TECs and attenuates the progression of kidney fibrosis, thus may hold therapeutic potential for the treatment of fibrotic kidney diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

