Nuclear compartmentalization of TERT mRNA and TUG1 lncRNA is driven by intron retention
- PMID: 34083519
- PMCID: PMC8175569
- DOI: 10.1038/s41467-021-23221-w
Nuclear compartmentalization of TERT mRNA and TUG1 lncRNA is driven by intron retention
Erratum in
-
Publisher Correction: Nuclear compartmentalization of TERT mRNA and TUG1 lncRNA is driven by intron retention.Nat Commun. 2021 Oct 21;12(1):6189. doi: 10.1038/s41467-021-26492-5. Nat Commun. 2021. PMID: 34675241 Free PMC article. No abstract available.
Abstract
The spatial partitioning of the transcriptome in the cell is an important form of gene-expression regulation. Here, we address how intron retention influences the spatio-temporal dynamics of transcripts from two clinically relevant genes: TERT (Telomerase Reverse Transcriptase) pre-mRNA and TUG1 (Taurine-Upregulated Gene 1) lncRNA. Single molecule RNA FISH reveals that nuclear TERT transcripts uniformly and robustly retain specific introns. Our data suggest that the splicing of TERT retained introns occurs during mitosis. In contrast, TUG1 has a bimodal distribution of fully spliced cytoplasmic and intron-retained nuclear transcripts. We further test the functionality of intron-retention events using RNA-targeting thiomorpholino antisense oligonucleotides to block intron excision. We show that intron retention is the driving force for the nuclear compartmentalization of these RNAs. For both RNAs, altering this splicing-driven subcellular distribution has significant effects on cell viability. Together, these findings show that stable retention of specific introns can orchestrate spatial compartmentalization of these RNAs within the cell. This process reveals that modulating RNA localization via targeted intron retention can be utilized for RNA-based therapies.
Conflict of interest statement
The authors declare no competing interests. T.R.C. is on the Merck board and is a consultant for Storm Therapeutics and Eikon Therapeutics.
Figures
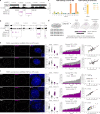
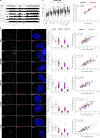

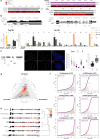

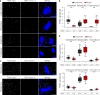

References
-
- Lécuyer E, et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–187. - PubMed
-
- Mauger O, Lemoine F, Scheiffele P. Targeted intron retention and excision for rapid gene regulation in response to neuronal activity. Neuron. 2016;92:1266–1278. - PubMed
-
- Chin A, Eric L. RNA localization: making its way to the center stage. Biochim. Biophys. Acta Gen. Subj. 2017;1861:2956–2970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

