Risk factors for rapid axial length elongation with low concentration atropine for myopia control
- PMID: 34083576
- PMCID: PMC8175344
- DOI: 10.1038/s41598-021-88719-1
Risk factors for rapid axial length elongation with low concentration atropine for myopia control
Abstract
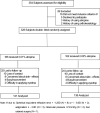
Three hundred and twenty-eight myopic children, randomized to use either 0.01% (N = 166) or 0.02% (N = 162) atropine were enrolled in this study. Gender, age, body mass index(BMI), parental myopia status, atropine concentration used, pupil diameter, amplitude of accommodation, spherical equivalent refractive error (SER), anterior chamber depth (ACD) and axial length (AL) were collected at baseline and 1 year after using atropine. Rapid AL elongation was defined as > 0.36 mm growth per year. Univariate analyses showed that children with rapid AL elongation tend to be younger, have a smaller BMI, use of 0.01% atropine, narrow ACD, lower SER, shorter AL, smaller change in pupil diameter between 1 year and baseline (all P < 0.05). Multivariate logistic regression analyses confirmed that rapid AL elongation was associated with children that were younger at baseline (P < 0.0001), use of 0.01% atropine (P = 0.04), a shorter baseline AL (P = 0.03) and a smaller change in pupil diameter between 1 year and baseline (P = 0.04). Younger children with shorter AL at baseline, less change in their pupil diameter with atropine treatment and using the lower of the two atropine concentrations may undergo rapid AL elongation over a 12 months myopia control treatment period.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources