Liver stiffness regression after sustained virological response by direct-acting antivirals reduces the risk of outcomes
- PMID: 34083617
- PMCID: PMC8175552
- DOI: 10.1038/s41598-021-91099-1
Liver stiffness regression after sustained virological response by direct-acting antivirals reduces the risk of outcomes
Abstract
The role of liver stiffness measurement (LSM) after sustained virological response (SVR) in HCV patients treated by direct-acting antivirals (DAAs) remains unclear. We aimed to evaluate LSM regression value after SVR and to identify risk factors associated with liver related complications (LRC) or death. This retrospective study analyzed patients with LSM ≥ 10 kPa with LSM by transient elastography pre-DAAs and post-SVR. Patients with previous hepatic decompensation were excluded. Medical records were reviewed to identify primary outcomes. Kaplan-Meier curves and time-to-event Cox proportional-hazard models were performed. 456 patients [65% female, 62 years (IQR 57-68)] were included. During a follow-up of 2.3 years (IQR 1.6-2.7), 28 patients developed 37 outcomes [rate = 29.0 (95% CI 20.0-42.0) per 1000 person-years]. The cumulative incidence of outcomes was significantly lower in patients who regressed LSM ≥ 20% [3.4% (95% CI 1.8-7.0) vs. 9.0% (5.5-14.5), p = 0.028]. In a multivariate Cox-model [HR(95% CI)], male gender [HR = 3.00 (1.30-6.95), p = 0.010], baseline albumin < 3.5 mg/dL [HR = 4.49 (1.95-10.34), p < 0.001] and baseline unfavorable Baveno-VI [HR = 4.72 (1.32-16.83), p = 0.017] were independently associated and LSM regression ≥ 20% after SVR had a trend to reduce the risk of LRC or death [HR = 0.45 (0.21-1.02), p = 0.058]. The use of simple parameters before DAAs and repetition of LSM post-SVR can identify patients with different risks for severe outcome after HCV eradication.
Conflict of interest statement
The authors declare no competing interests.
Figures
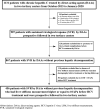
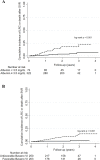
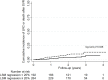
Similar articles
-
Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals.J Hepatol. 2020 Mar;72(3):472-480. doi: 10.1016/j.jhep.2019.10.005. Epub 2019 Oct 17. J Hepatol. 2020. PMID: 31629779
-
Effectiveness of direct-acting agents for hepatitis C and liver stiffness changing after sustained virological response.J Gastroenterol Hepatol. 2019 Dec;34(12):2187-2195. doi: 10.1111/jgh.14707. Epub 2019 Jun 26. J Gastroenterol Hepatol. 2019. PMID: 31062880
-
Impact of sustained virological response with DAAs on gastroesophageal varices and Baveno criteria in HCV-cirrhotic patients.J Gastroenterol. 2020 Feb;55(2):205-216. doi: 10.1007/s00535-019-01619-0. Epub 2019 Sep 6. J Gastroenterol. 2020. PMID: 31493238
-
Impact of liver-stiffness measurement on hepatocellular carcinoma development in chronic hepatitis C patients treated with direct-acting antivirals: A systematic review and time-to-event meta-analysis.J Gastroenterol Hepatol. 2021 Mar;36(3):601-608. doi: 10.1111/jgh.15243. Epub 2020 Sep 10. J Gastroenterol Hepatol. 2021. PMID: 32875681
-
Residual risk of liver disease after hepatitis C virus eradication.J Hepatol. 2021 Apr;74(4):952-963. doi: 10.1016/j.jhep.2020.11.040. Epub 2020 Dec 1. J Hepatol. 2021. PMID: 33276027 Review.
Cited by
-
Are We on the Right Track for HCV Micro-Elimination? HCV Management Practices in Dialysis Centers in Poland-A National Cross-Sectional Survey.J Clin Med. 2023 Apr 4;12(7):2711. doi: 10.3390/jcm12072711. J Clin Med. 2023. PMID: 37048794 Free PMC article.
-
Liver Fibrosis: From Basic Science towards Clinical Progress, Focusing on the Central Role of Hepatic Stellate Cells.Int J Mol Sci. 2024 Jul 18;25(14):7873. doi: 10.3390/ijms25147873. Int J Mol Sci. 2024. PMID: 39063116 Free PMC article. Review.
-
Evaluation of Long-Term Outcomes of Direct Acting Antiviral Agents in Chronic Kidney Disease Subjects: A Single Center Cohort Study.J Clin Med. 2023 May 17;12(10):3513. doi: 10.3390/jcm12103513. J Clin Med. 2023. PMID: 37240622 Free PMC article.
-
Prognostic Effects of Liver Fibrosis and Steatosis Determined Using Transient Elastography in Patients with Chronic Hepatitis B or C.Dig Dis Sci. 2023 Jun;68(6):2747-2756. doi: 10.1007/s10620-023-07943-z. Epub 2023 Apr 18. Dig Dis Sci. 2023. PMID: 37071242
-
Impact of direct-acting antiviral regimens on hepatic and extrahepatic manifestations of hepatitis C virus infection.World J Hepatol. 2022 Jun 27;14(6):1053-1073. doi: 10.4254/wjh.v14.i6.1053. World J Hepatol. 2022. PMID: 35978668 Free PMC article. Review.
References
-
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol.69(2), 461–511 10.1016/j.jhep.2018.03.026 (2018). - PubMed
-
- Ghany MG, Morgan TR, Panel A-IHCG Hepatitis C guidance 2019 update: American Association for the study of liver diseases-infectious diseases society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020;71(2):686–721. doi: 10.1002/hep.31060. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

