Alpha-synuclein increases in rodent and human spinal cord injury and promotes inflammation and tissue loss
- PMID: 34083630
- PMCID: PMC8175699
- DOI: 10.1038/s41598-021-91116-3
Alpha-synuclein increases in rodent and human spinal cord injury and promotes inflammation and tissue loss
Abstract
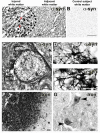
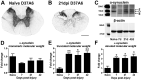
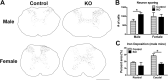
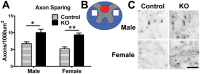
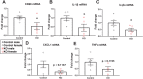
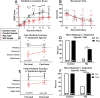
Synucleinopathies are neurodegenerative diseases in which α-synuclein protein accumulates in neurons and glia. In these diseases, α-synuclein forms dense intracellular aggregates that are disease hallmarks and actively contribute to tissue pathology. Interestingly, many pathological mechanisms, including iron accumulation and lipid peroxidation, are shared between classical synucleinopathies such as Alzheimer's disease, Parkinson's disease and traumatic spinal cord injury (SCI). However, to date, no studies have determined if α-synuclein accumulation occurs after human SCI. To examine this, cross-sections from injured and non-injured human spinal cords were immunolabeled for α-synuclein. This showed robust α-synuclein accumulation in profiles resembling axons and astrocytes in tissue surrounding the injury, revealing that α-synuclein markedly aggregates in traumatically injured human spinal cords. We also detected significant iron deposition in the injury site, a known catalyst for α-synuclein aggregation. Next a rodent SCI model mimicking the histological features of human SCI revealed aggregates and structurally altered monomers of α-synuclein are present after SCI. To determine if α-synuclein exacerbates SCI pathology, α-synuclein knockout mice were tested. Compared to wild type mice, α-synuclein knockout mice had significantly more spared axons and neurons and lower pro-inflammatory mediators, macrophage accumulation, and iron deposition in the injured spinal cord. Interestingly, locomotor analysis revealed that α-synuclein may be essential for dopamine-mediated hindlimb function after SCI. Collectively, the marked upregulation and long-lasting accumulation of α-synuclein and iron suggests that SCI may fit within the family of synucleinopathies and offer new therapeutic targets for promoting neuron preservation and improving function after spinal trauma.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Lentivirus-mediated downregulation of α-synuclein reduces neuroinflammation and promotes functional recovery in rats with spinal cord injury.J Neuroinflammation. 2019 Dec 30;16(1):283. doi: 10.1186/s12974-019-1658-2. J Neuroinflammation. 2019. PMID: 31888724 Free PMC article.
-
Transcriptomic analysis of α-synuclein knockdown after T3 spinal cord injury in rats.BMC Genomics. 2019 Nov 14;20(1):851. doi: 10.1186/s12864-019-6244-6. BMC Genomics. 2019. PMID: 31726970 Free PMC article.
-
SARM1 promotes neuroinflammation and inhibits neural regeneration after spinal cord injury through NF-κB signaling.Theranostics. 2021 Feb 20;11(9):4187-4206. doi: 10.7150/thno.49054. eCollection 2021. Theranostics. 2021. PMID: 33754056 Free PMC article.
-
Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury.Int J Mol Sci. 2021 Dec 17;22(24):13577. doi: 10.3390/ijms222413577. Int J Mol Sci. 2021. PMID: 34948371 Free PMC article. Review.
-
Inflammatory pathways in spinal cord injury.Int Rev Neurobiol. 2012;106:127-52. doi: 10.1016/B978-0-12-407178-0.00006-5. Int Rev Neurobiol. 2012. PMID: 23211462 Review.
Cited by
-
Risk of Parkinson's disease in spinal cord injury: a nationwide cohort study in South Korea.Sci Rep. 2024 Oct 3;14(1):23016. doi: 10.1038/s41598-024-74103-2. Sci Rep. 2024. PMID: 39362952 Free PMC article.
-
Astrocyte alterations in α-synucleinopathies.Front Cell Neurosci. 2025 Aug 6;19:1650326. doi: 10.3389/fncel.2025.1650326. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40842565 Free PMC article. Review.
-
Chaperone-Mediated Autophagy in Neurodegenerative Diseases and Acute Neurological Insults in the Central Nervous System.Cells. 2022 Apr 2;11(7):1205. doi: 10.3390/cells11071205. Cells. 2022. PMID: 35406769 Free PMC article. Review.
-
Exploring α-Syn's Functions Through Ablation Models: Physiological and Pathological Implications.Cell Mol Neurobiol. 2025 May 19;45(1):44. doi: 10.1007/s10571-025-01560-2. Cell Mol Neurobiol. 2025. PMID: 40389720 Free PMC article. Review.
-
Catalpol as a Component of Rehmannia glutinosa Protects Spinal Cord Injury by Inhibiting Endoplasmic Reticulum Stress-Mediated Neuronal Apoptosis.Front Pharmacol. 2022 Jul 8;13:860757. doi: 10.3389/fphar.2022.860757. eCollection 2022. Front Pharmacol. 2022. PMID: 35873542 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

