Extended-release naltrexone/bupropion is safe and effective among subjects with type 2 diabetes already taking incretin agents: a post-hoc analysis of the LIGHT trial
- PMID: 34083744
- PMCID: PMC8310797
- DOI: 10.1038/s41366-021-00831-4
Extended-release naltrexone/bupropion is safe and effective among subjects with type 2 diabetes already taking incretin agents: a post-hoc analysis of the LIGHT trial
Abstract
Background: Extended-release naltrexone/bupropion (NB) is indicated for chronic weight management. Incretin agents are recommended for patients with type 2 diabetes. This analysis looked at the add-on of NB to incretins to see if weight loss could occur in patients already stabilized on incretin agents.
Methods: This was a post-hoc analysis of NB vs. placebo (PL) among subjects with type 2 diabetes stable on an incretin agent prior to randomization in a double-blind, PL-controlled cardiovascular outcome trial (N = 1317).
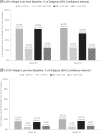
Results: Over 1 year, mean weight loss was significantly greater among NB patients vs. PL among those taking DPP-4i (mean absolute difference 4.6% [p < 0.0001]) and those taking GLP-1RAs (mean absolute difference 5.2%, p < 0.0001). Proportions of subjects achieving 5% weight loss were significantly greater for NB vs. PL at weeks 26 and 52 among those taking DPP-4is or GLP-1RAs. There were no significant differences in effectiveness observed between NB + DPP-4i and NB + GLP-1RA or between PL + DPP-4i and PL + GLP-1RA in any of the analyses. Serious adverse events were reported by 9.1% and 11.1% for PL + DPP-4i and PL + GLP-1RA, respectively, and 13.3% and 12.4% of NB + DPP-4i and NB + GLP-1RA, respectively.
Conclusion: NB appears to be effective in reducing weight in patients with T2DM and obesity/overweight who are taking DPP-4ihibitors or GLP-1RA. The SAE rates in all arms of this analysis were lower than have been reported in other cardiovascular outcome trials in type 2 diabetes.
© 2021. The Author(s).
Conflict of interest statement
SW is the owner and director of Wharton Medical Clinic (WMC). He has previously received funding in the form of grants for research from the Canadian Institutes of Health Research and Mitacs. He has also received funding from Novo Nordisk, Bausch Health Canada Inc., Eli Lilly and Company, Janssen Pharmaceuticals, and AstraZeneca for advisory work. EK and RAGC are currently employed by the Wharton Medical Clinic. PY is an employee of Bausch Health Companies. MB (Melonie Burrows) is a former employee of Bausch Health Companies. JB and MB (Maxime Barakat) are employees of, and shareholders in, Bausch Health Companies. FC received consulting fees from Bausch Health, Abbvie Corporation, and Janssen Inc. EG is an employee of Currax Pharmaceuticals LLC.
Figures


References
-
- Wing RR. Weight loss in the management of type 2 diabetes. In: Gerstein HC, Hyanes B, editors. Evidence-based diabetes care. Hamilton. B.C. Decker Inc.; 2000. pp. 252–76.
-
- Diabetes Canada Clinical Practice Guidelines Expert Committee, Wharton S, Pedersen SD, Lau DCW, Sharma AM. Weight management in diabetes. Can J Diabetes. 2018;42(Suppl 1):S124–9. - PubMed
-
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

