SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus
- PMID: 34083759
- PMCID: PMC8175744
- DOI: 10.1038/s42003-021-02220-z
SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus
Abstract
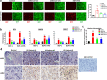
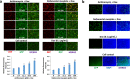
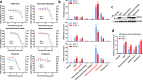
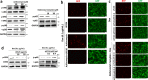
An outbreak of the novel coronavirus SARS-CoV-2, the causative agent of Coronavirus Disease-2019 (COVID-19), a respiratory disease, has infected almost one hundred million people since the end of 2019, killed over two million, and caused worldwide social and economic disruption. Because the mechanisms of SARS-CoV-2 infection of host cells and its pathogenesis remain largely unclear, there are currently no antiviral drugs with proven efficacy. Besides severe respiratory and systematic symptoms, several comorbidities increase risk of fatal disease outcome. Therefore, it is required to investigate the impacts of COVID-19 on pre-existing diseases of patients, such as cancer and other infectious diseases. In the current study, we report that SARS-CoV-2 encoded proteins and some currently used anti-COVID-19 drugs are able to induce lytic reactivation of Kaposi's sarcoma-associated herpesvirus (KSHV), one of major human oncogenic viruses, through manipulation of intracellular signaling pathways. Our data indicate that those KSHV + patients especially in endemic areas exposure to COVID-19 or undergoing the treatment may have increased risks to develop virus-associated cancers, even after they have fully recovered from COVID-19.
Conflict of interest statement
The authors declare no competing interests.
Figures
Update of
-
SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus.bioRxiv [Preprint]. 2020 Oct 2:2020.10.02.324228. doi: 10.1101/2020.10.02.324228. bioRxiv. 2020. Update in: Commun Biol. 2021 Jun 3;4(1):682. doi: 10.1038/s42003-021-02220-z. PMID: 33024968 Free PMC article. Updated. Preprint.
References
-
- Elezkurtaj, S. et al. Causes of death and comorbidities in patients with COVID-19. medRxiv10.1101/2020.06.15.20131540 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

