Scalable, multimodal profiling of chromatin accessibility, gene expression and protein levels in single cells
- PMID: 34083792
- PMCID: PMC8763625
- DOI: 10.1038/s41587-021-00927-2
Scalable, multimodal profiling of chromatin accessibility, gene expression and protein levels in single cells
Abstract
Recent technological advances have enabled massively parallel chromatin profiling with scATAC-seq (single-cell assay for transposase accessible chromatin by sequencing). Here we present ATAC with select antigen profiling by sequencing (ASAP-seq), a tool to simultaneously profile accessible chromatin and protein levels. Our approach pairs sparse scATAC-seq data with robust detection of hundreds of cell surface and intracellular protein markers and optional capture of mitochondrial DNA for clonal tracking, capturing three distinct modalities in single cells. ASAP-seq uses a bridging approach that repurposes antibody:oligonucleotide conjugates designed for existing technologies that pair protein measurements with single-cell RNA sequencing. Together with DOGMA-seq, an adaptation of CITE-seq (cellular indexing of transcriptomes and epitopes by sequencing) for measuring gene activity across the central dogma of gene regulation, we demonstrate the utility of systematic multi-omic profiling by revealing coordinated and distinct changes in chromatin, RNA and surface proteins during native hematopoietic differentiation and peripheral blood mononuclear cell stimulation and as a combinatorial decoder and reporter of multiplexed perturbations in primary T cells.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
COMPETING INTERESTS
C.A.L., L.S.L., V.G.S., and A.R. are listed as co-inventors on a patent related to mtscATAC-seq (US provisional patent application 62/683,502). In the past three years, RS has worked as a consultant for Bristol-Myers Squibb, Regeneron, and Kallyope, and served as an SAB member for Immunai and Resolve BioSciences. A.R. is a founder and equity holder of Celsius Therapeutics, an equity holder in Immunitas Therapeutics and until August 31, 2020 was an SAB member of Syros Pharmaceuticals, Neogene Therapeutics, Asimov and ThermoFisher Scientific. From August 1, 2020, A.R. is an employee of Genentech. P.S. is listed as co-inventor on a patent related to this work (US provisional patent application 62/515-180).
Figures







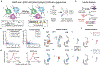
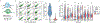





Comment in
-
Arsenal of single-cell multi-omics methods expanded.Nat Methods. 2021 Aug;18(8):858. doi: 10.1038/s41592-021-01245-w. Nat Methods. 2021. PMID: 34354288 No abstract available.
References
-
- Zhu C, Preissl S & Ren B Single-cell multimodal omics: the power of many. Nat. Methods 17, 11–14 (2020). - PubMed
-
- Schier AF Single-cell biology: beyond the sum of its parts. Nat. Methods 17, 17–20 (2020). - PubMed
-
- Stuart T & Satija R Integrative single-cell analysis. Nat. Rev. Genet 20, 257–272 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

