CD40 Activity on Mesenchymal Cells Negatively Regulates OX40L to Maintain Bone Marrow Immune Homeostasis Under Stress Conditions
- PMID: 34084166
- PMCID: PMC8168593
- DOI: 10.3389/fimmu.2021.662048
CD40 Activity on Mesenchymal Cells Negatively Regulates OX40L to Maintain Bone Marrow Immune Homeostasis Under Stress Conditions
Abstract
Background: Within the bone marrow (BM), mature T cells are maintained under homeostatic conditions to facilitate proper hematopoietic development. This homeostasis depends upon a peculiar elevated frequency of regulatory T cells (Tregs) and immune regulatory activities from BM-mesenchymal stem cells (BM-MSCs). In response to BM transplantation (BMT), the conditioning regimen exposes the BM to a dramatic induction of inflammatory cytokines and causes an unbalanced T-effector (Teff) and Treg ratio. This imbalance negatively impacts hematopoiesis, particularly in regard to B-cell lymphopoiesis that requires an intact cross-talk between BM-MSCs and Tregs. The mechanisms underlying the ability of BM-MSCs to restore Treg homeostasis and proper B-cell development are currently unknown.
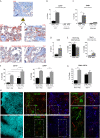
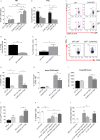
Methods: We studied the role of host radio-resistant cell-derived CD40 in restoring Teff/Treg homeostasis and proper B-cell development in a murine model of BMT. We characterized the host cellular source of CD40 and performed radiation chimera analyses by transplanting WT or Cd40-KO with WT BM in the presence of T-reg and co-infusing WT or - Cd40-KO BM-MSCs. Residual host and donor T cell expansion and activation (cytokine production) and also the expression of Treg fitness markers and conversion to Th17 were analyzed. The presence of Cd40+ BM-MSCs was analyzed in a human setting in correlation with the frequency of B-cell precursors in patients who underwent HSCT and variably developed acute graft-versus-host (aGVDH) disease.
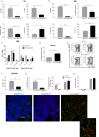
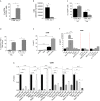
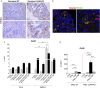
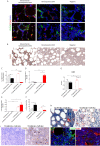
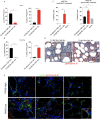
Results: CD40 expression is nearly undetectable in the BM, yet a Cd40-KO recipient of WT donor chimera exhibited impaired B-cell lymphopoiesis and Treg development. Lethal irradiation promotes CD40 and OX40L expression in radio-resistant BM-MSCs through the induction of pro-inflammatory cytokines. OX40L favors Teff expansion and activation at the expense of Tregs; however, the expression of CD40 dampens OX40L expression and restores Treg homeostasis, thus facilitating proper B-cell development. Indeed, in contrast to dendritic cells in secondary lymphoid organs that require CD40 triggers to express OX40L, BM-MSCs require CD40 to inhibit OX40L expression.
Conclusions: CD40+ BM-MSCs are immune regulatory elements within BM. Loss of CD40 results in uncontrolled T cell activation due to a reduced number of Tregs, and B-cell development is consequently impaired. GVHD provides an example of how a loss of CD40+ BM-MSCs and a reduction in B-cell precursors may occur in a human setting.
Keywords: B-cell development; CD40; OX40L; bone marrow transplantation; mesenchymal cell.
Copyright © 2021 Bassani, Tripodo, Portararo, Gulino, Botti, Chiodoni, Jachetti, Bolli, Ciciarello, Joehrens, Anagnostopoulos, Na, Curti, Colombo and Sangaletti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

