Current clinical trials and patent update on lung cancer: a retrospective review
- PMID: 34084211
- PMCID: PMC8162165
- DOI: 10.2217/lmt-2020-0029
Current clinical trials and patent update on lung cancer: a retrospective review
Abstract
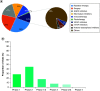
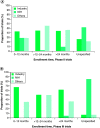
Several clinical trials using different interventions are currently being sponsored to combat lung cancer at its different stages. The purpose of this study was to provide a portfolio of those trials. All active, open and recruiting clinical trials registered at ClinicalTrials.gov up to March 2018 were included. Information related to 6092 registered lung cancer trials was downloaded. Phase II trials were in the majority, comprising nearly 48.7% of total clinical trials with industry the major sponsor (41.3%) followed by NIH (12.3%). Multicenter studies were the norm accounting for 47.9% and the main study location was the USA (50.9%). Common interventions were radiation (26%), surgery (22%) and EGFR inhibitors (17%). Patent information includes major patent filing office and sponsors. The data analysis provides a comprehensive description of lung cancer trials.
Keywords: chemotherapy; clinical trials; clinicaltrials.gov; lung cancer; lung cancer patents.
© 2021 Shrikant Pawar.
Conflict of interest statement
Financial & competing interests disclosure The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending or royalties. No writing assistance was utilized in the production of this manuscript.
Figures
Similar articles
-
INGN 201: Ad-p53, Ad5CMV-p53, adenoviral p53, p53 gene therapy--introgen, RPR/INGN 201.Drugs R D. 2007;8(3):176-87. doi: 10.2165/00126839-200708030-00005. Drugs R D. 2007. PMID: 17472413 Review.
-
INGN 201: Ad-p53, Ad5CMV-p53, Adenoviral p53, INGN 101, p53 gene therapy--Introgen, RPR/INGN 201.BioDrugs. 2003;17(3):216-22. doi: 10.2165/00063030-200317030-00010. BioDrugs. 2003. PMID: 12749760 Review.
-
Assessment of Trends in the Design, Accrual, and Completion of Trials Registered in ClinicalTrials.gov by Sponsor Type, 2000-2019.JAMA Netw Open. 2020 Aug 3;3(8):e2014682. doi: 10.1001/jamanetworkopen.2020.14682. JAMA Netw Open. 2020. PMID: 32845329 Free PMC article.
-
Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010.JAMA. 2012 May 2;307(17):1838-47. doi: 10.1001/jama.2012.3424. JAMA. 2012. PMID: 22550198
-
Current status and perspectives of interventional clinical trials for glioblastoma - analysis of ClinicalTrials.gov.Radiat Oncol. 2017 Jan 3;12(1):1. doi: 10.1186/s13014-016-0740-5. Radiat Oncol. 2017. PMID: 28049492 Free PMC article. Review.
References
-
- Key Statistics for Lung Cancer. American Cancer Society. (2021). https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
-
- Sateia HF, Choi Y, Stewart RW et al. Screening for lung cancer. Semin. Oncol. 44(1), 74–82 (2017). - PubMed
-
- Cancer Research UK. Cancer statistics reports for the UK. (2018). http://www.cancerresearchuk.org/aboutcancer/statistics/cancerstatsreport/
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
