Challenges in bioinformatics approaches to tumor mutation burden analysis
- PMID: 34084222
- PMCID: PMC8161416
- DOI: 10.3892/ol.2021.12816
Challenges in bioinformatics approaches to tumor mutation burden analysis
Abstract
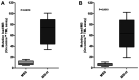
Several immune checkpoint inhibitors (ICIs) have already been introduced into clinical practice or are in advanced phases of clinical experimentation. Extensive efforts are being made to identify robust biomarkers to select patients who may benefit from treatment with ICIs. Tumor mutation burden (TMB) may be a relevant biomarker of response to ICIs in different tumor types; however, its clinical use is challenged by the analytical methods required for its evaluation. The possibility of using targeted next-generation sequencing panels has been investigated as an alternative to the standard whole exome sequencing approach. However, no standardization exists in terms of genes covered, types of mutations included in the estimation of TMB, bioinformatics pipelines for data analysis, and cut-offs used to discriminate samples with high, intermediate or low TMB. Bioinformatics serve a relevant role in the analysis of targeted sequencing data and its standardization is essential to deliver a reliable test in clinical practice. In the present study, cultured and formalin-fixed, paraffin-embedded cell lines were analyzed using a commercial panel for TMB testing; the results were compared with data from the literature and public databases, demonstrating a good correlation. Additionally, the correlation between high tumor mutation burden and microsatellite instability was confirmed. The bioinformatics analyses were conducted using two different pipelines to highlight the challenges associated with the development of an appropriate analytical workflow.
Keywords: DNA mutational analysis; immunotherapy; molecular pathology; next-generation sequencing; tumor mutation burden.
Copyright: © Fenizia et al.
Conflict of interest statement
NN has received speaker's fee and is on the advisory boards of the following companies: Astra-Zeneca, Bayer, Illumina, Incyte, MSD, Merck KgA, J&J, Qiagen, Roche, Thermo Fisher Scientific, Inc. CA, FH and RC are Thermo Fisher Scientific, Inc. employees. Thermo Fisher Scientific, Inc. is the supplier of the Oncomine Tumor Mutation Load assay used within the study. The other authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
