Photodynamic viral inactivation: Recent advances and potential applications
- PMID: 34084253
- PMCID: PMC8132927
- DOI: 10.1063/5.0044713
Photodynamic viral inactivation: Recent advances and potential applications
Abstract
Antibiotic-resistant bacteria, which are growing at a frightening rate worldwide, has put the world on a long-standing alert. The COVID-19 health crisis reinforced the pressing need to address a fast-developing pandemic. To mitigate these health emergencies and prevent economic collapse, cheap, practical, and easily applicable infection control techniques are essential worldwide. Application of light in the form of photodynamic action on microorganisms and viruses has been growing and is now successfully applied in several areas. The efficacy of this approach has been demonstrated in the fight against viruses, prompting additional efforts to advance the technique, including safety use protocols. In particular, its application to suppress respiratory tract infections and to provide decontamination of fluids, such as blood plasma and others, can become an inexpensive alternative strategy in the fight against viral and bacterial infections. Diverse early treatment methods based on photodynamic action enable an accelerated response to emerging threats prior to the availability of preventative drugs. In this review, we evaluate a vast number of photodynamic demonstrations and first-principle proofs carried out on viral control, revealing its potential and encouraging its rapid development toward safe clinical practice. This review highlights the main research trends and, as a futuristic exercise, anticipates potential situations where photodynamic treatment can provide a readily available solution.
© 2021 Author(s).
Figures
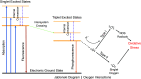
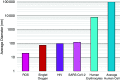

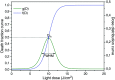
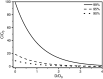


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
