Chemical modification of the adeno-associated virus capsid to improve gene delivery
- PMID: 34084369
- PMCID: PMC8145868
- DOI: 10.1039/c9sc04189c
Chemical modification of the adeno-associated virus capsid to improve gene delivery
Abstract
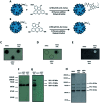
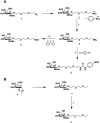
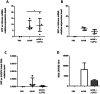
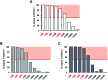
Gene delivery vectors based on adeno-associated virus (AAV) are highly promising due to several desirable features of this parent virus, including a lack of pathogenicity, efficient infection of dividing and non-dividing cells and sustained maintenance of the viral genome. However, the conclusion from clinical data using these vectors is that there is a need to develop new AAVs with a higher transduction efficiency and specificity for relevant target tissues. To overcome these limitations, we chemically modified the surface of the capsid of AAV vectors. These modifications were achieved by chemical coupling of a ligand by the formation of a thiourea functionality between the amino group of the capsid proteins and the reactive isothiocyanate motif incorporated into the ligand. This strategy does not require genetic engineering of the capsid sequence. The proof of concept was first evidenced using a fluorophore (FITC). Next, we coupled the N-acetylgalactosamine ligand onto the surface of the AAV capsid for asialoglycoprotein receptor-mediated hepatocyte-targeted delivery. Chemically-modified capsids also showed reduced interactions with neutralizing antibodies. Taken together, our findings reveal the possibility of creating a specific engineered platform for targeting AAVs via chemical coupling.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
M. M., D. D., are E. A. are inventors on a patent including the technology described in this manuscript. JPC and GGA are employees of Vivet Therapeutics.
Figures







Similar articles
-
Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer.Pharm Res. 2008 Mar;25(3):489-99. doi: 10.1007/s11095-007-9431-0. Epub 2007 Sep 1. Pharm Res. 2008. PMID: 17763830 Free PMC article. Review.
-
Synthetically Engineered Adeno-Associated Virus for Efficient, Safe, and Versatile Gene Therapy Applications.ACS Nano. 2020 Nov 24;14(11):14262-14283. doi: 10.1021/acsnano.0c03850. Epub 2020 Oct 19. ACS Nano. 2020. PMID: 33073995 Review.
-
Rational engineering of adeno-associated virus capsid enhances human hepatocyte tropism and reduces immunogenicity.Cell Prolif. 2022 Dec;55(12):e13339. doi: 10.1111/cpr.13339. Epub 2022 Sep 22. Cell Prolif. 2022. PMID: 36135100 Free PMC article.
-
Generation of Targeted Adeno-Associated Virus (AAV) Vectors for Human Gene Therapy.Curr Pharm Des. 2015;21(22):3248-56. doi: 10.2174/1381612821666150531171653. Curr Pharm Des. 2015. PMID: 26027561 Review.
-
Characterization of the Adeno-Associated Virus 1 and 6 Sialic Acid Binding Site.J Virol. 2016 May 12;90(11):5219-5230. doi: 10.1128/JVI.00161-16. Print 2016 Jun 1. J Virol. 2016. PMID: 26962225 Free PMC article.
Cited by
-
Intravitreal air tamponade after AAV2 subretinal injection modifies retinal EGFP distribution.Mol Ther Methods Clin Dev. 2023 Feb 15;28:387-393. doi: 10.1016/j.omtm.2023.02.006. eCollection 2023 Mar 9. Mol Ther Methods Clin Dev. 2023. PMID: 36874242 Free PMC article.
-
Progress in AAV-Mediated In Vivo Gene Therapy and Its Applications in Central Nervous System Diseases.Int J Mol Sci. 2025 Feb 28;26(5):2213. doi: 10.3390/ijms26052213. Int J Mol Sci. 2025. PMID: 40076831 Free PMC article. Review.
-
Towards Clinical Implementation of Adeno-Associated Virus (AAV) Vectors for Cancer Gene Therapy: Current Status and Future Perspectives.Cancers (Basel). 2020 Jul 14;12(7):1889. doi: 10.3390/cancers12071889. Cancers (Basel). 2020. PMID: 32674264 Free PMC article. Review.
-
Fantastic AAV Gene Therapy Vectors and How to Find Them-Random Diversification, Rational Design and Machine Learning.Pathogens. 2022 Jul 3;11(7):756. doi: 10.3390/pathogens11070756. Pathogens. 2022. PMID: 35890005 Free PMC article. Review.
-
Precise Manipulation of the Site and Stoichiometry of Capsid Modification Enables Optimization of Functional Adeno-Associated Virus Conjugates.Bioconjug Chem. 2024 Jan 17;35(1):64-71. doi: 10.1021/acs.bioconjchem.3c00411. Epub 2023 Dec 16. Bioconjug Chem. 2024. PMID: 38103182 Free PMC article.
References
-
- Russell S. Bennett J. Wellman J. A. Chung D. C. Yu Z. F. Tillman A. Wittes J. Pappas J. Elci O. McCague S. Cross D. Marshall K. A. Walshire J. Kehoe T. L. Reichert H. Davis M. Raffini L. George L. A. Hudson F. P. Dingfield L. Zhu X. Haller J. A. Sohn E. H. Mahajan V. B. Pfeifer W. Weckmann M. Johnson C. Gewaily D. Drack A. Stone E. Wachtel K. Simonelli F. Leroy B. P. Wright J. F. High K. A. Maguire A. M. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

