Sulfatase-cleavable linkers for antibody-drug conjugates
- PMID: 34084399
- PMCID: PMC8157321
- DOI: 10.1039/c9sc06410a
Sulfatase-cleavable linkers for antibody-drug conjugates
Abstract
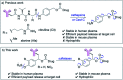
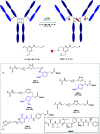
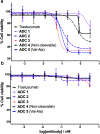
Antibody-drug conjugates (ADCs) are a class of targeted drug delivery agents combining the cell-selectivity of monoclonal antibodies (mAbs) and the cytotoxicity of small molecules. These two components are joined by a covalent linker, whose nature is critical to the efficacy and safety of the ADC. Enzyme-cleavable dipeptidic linkers have emerged as a particularly effective ADC linker type due to their ability to selectively release the payload in the lysosomes of target cells. However, these linkers have a number of drawbacks, including instability in rodent plasma and their inherently high hydrophobicity. Here we show that arylsulfate-containing ADC linkers are cleaved by lysosomal sulfatase enzymes to tracelessly release their payload, while circumventing the instability problems associated with dipeptide-linkers. When incorporated with trastuzumab and the highly potent monomethyl auristatin E (MMAE) payload, the arylsulfate-containing ADC 2 and ADC 3 were more cytotoxic than the non-cleavable ADC 4 against HER2-positive cells, while maintaining selectivity over HER2-negative cells. We propose that the stability, solubility and synthetic tractability of our arylsulfate linkers make them an attractive new motif for cleavable ADC linkers, with clear benefits over the widely used dipeptidic linkers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

