Non-stackable molecules assemble into porous crystals displaying concerted cavity-changing motions
- PMID: 34084437
- PMCID: PMC8115244
- DOI: 10.1039/d1sc01163d
Non-stackable molecules assemble into porous crystals displaying concerted cavity-changing motions
Abstract
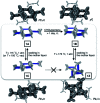
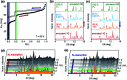
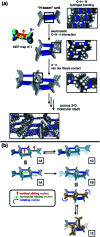
With small molecules, it is not easy to create large void spaces. Flat aromatics stack tightly, while flexible chains fold to fill the cavities. As an intuitive design to make open channels inside molecularly constructed solids, we employed propeller-shaped bicyclic triazoles to prepare a series of aromatic-rich three-dimensional (3D) building blocks. This modular approach has no previous example, but is readily applicable to build linear, bent, and branched arrays of non-stackable architectural motifs from existing flat aromatics by single-pot reactions. A letter H-shaped molecule thus prepared self-assembles into porous crystals, the highly unusual stepwise gas sorption behaviour of which prompted in-depth studies. A combination of single-crystal and powder X-ray diffraction analysis revealed multiple polymorphs, and sterically allowed pathways for their reversible interconversions that open and close the pores in response to external stimuli.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Das S. Heasman P. Ben T. Qiu S. Chem. Rev. 2017;117:1515–1563. - PubMed
-
- Holst J. R. Trewin A. Cooper A. I. Nat. Chem. 2010;2:915–920. - PubMed
-
- Tozawa T. Jones J. T. A. Swamy S. I. Jiang S. Adams D. J. Shakespeare S. Clowes R. Bradshaw D. Hasell T. Chong S. Y. Tang C. Thompson S. Parker J. Trewin A. Bacsa J. Slawin A. M. Z. Steiner A. Cooper A. I. Nat. Mater. 2009;8:973–978. - PubMed
-
- Chong J. H. Ardakani S. J. Smith K. J. MacLachlan M. J. Chem.–Eur. J. 2009;15:11824–11828. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

