Photocatalytic decarboxylative amidosulfonation enables direct transformation of carboxylic acids to sulfonamides
- PMID: 34084443
- PMCID: PMC8115300
- DOI: 10.1039/d1sc01389k
Photocatalytic decarboxylative amidosulfonation enables direct transformation of carboxylic acids to sulfonamides
Abstract
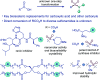
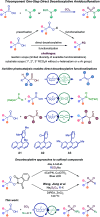
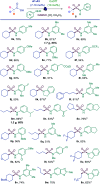
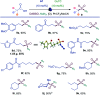
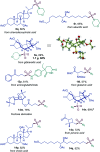
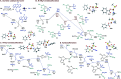
Sulfonamides feature prominently in organic synthesis, materials science and medicinal chemistry, where they play important roles as bioisosteric replacements of carboxylic acids and other carbonyls. Yet, a general synthetic platform for the direct conversion of carboxylic acids to a range of functionalized sulfonamides has remained elusive. Herein, we present a visible light-induced, dual catalytic platform that for the first time allows for a one-step access to sulfonamides and sulfonyl azides directly from carboxylic acids. The broad scope of the direct decarboxylative amidosulfonation (DDAS) platform is enabled by the efficient direct conversion of carboxylic acids to sulfinic acids that is catalyzed by acridine photocatalysts and interfaced with copper-catalyzed sulfur-nitrogen bond-forming cross-couplings with both electrophilic and nucleophilic reagents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- The Chemistry of Sulphinic Acids, Esters and their Derivatives, ed. S. Patai, John Wiley & Sons, Ltd, New Jersey, 1990
- The Chemistry of Sulphonic Acids, Esters and their Derivatives, ed. S. Patai and Z. Rapport, John Wiley & Sons, New York, 1991
- Zhao X. Dimitrijević E. Dong V. M. J. Am. Chem. Soc. 2009;131:3466–3467. - PubMed
- Dornan P. K. Kou K. G. M. Houk K. N. Dong V. M. J. Am. Chem. Soc. 2014;136:291. - PMC - PubMed
- García-Domínguez A. Müller S. Nevado C. Angew. Chem., Int. Ed. 2017;56:9949–9952. - PubMed
- Burke E. G. Gold B. Hoang T. T. Raines R. T. Schomaker J. M. J. Am. Chem. Soc. 2017;139:8029–8037. - PMC - PubMed
- Nguyen V. T. Dang H. T. Pham H. H. Nguyen V. D. Flores-Hansen C. Arman H. D. Larionov O. V. J. Am. Chem. Soc. 2018;140:8434–8438. - PMC - PubMed
- Ratushnyy M. Kamenova M. Gevorgyan V. Chem. Sci. 2018;9:7193–7197. - PMC - PubMed
- Hell S. M. Meyer C. F. Misale A. Sap J. B. I. Christensen K. K. Willis M. C. Trabanco A. A. Gouverneur V. Angew. Chem., Int. Ed. 2020;59:11620–11626. - PMC - PubMed
- Nambo M. Tahara Y. Yim J. C.-H. Yokogawa D. Crudden C. M. Chem. Sci. 2021 doi: 10.1039/D1SC00133G. - DOI - PMC - PubMed
-
- Patani G. A. LaVoie E. J. Chem. Rev. 1996;96:3147–3176. - PubMed
- Metabolism, Pharmacokinetics and Toxicity of Functional Groups, ed. D. A. Smith, Royal Society of Chemistry: London, United Kingdom, 2010, pp. 99–167, 210–274
- Ilardi E. A. Vitaku E. Njardarson J. T. J. Med. Chem. 2014;57:2832–2842. - PubMed
- Scott K. A. Njardarson J. T. Top. Curr. Chem. 2018;376:5. - PubMed
-
- Friedman H. L., Influence of isosteric replacements upon biological activity, NAS-NRS, Washington, DC, 1951, vol. 206, pp. 295–358, NAS-NRS Publication No. 206
-
-
For examples of non-decarboxylative one-step intermolecular tricomponent approaches to aromatic sulfonamides, see:
- Nguyen B. Emmett E. J. Willis M. C. J. Am. Chem. Soc. 2010;132:16372–16373. - PubMed
- Tsai A. S. Curto J. M. Rocke B. N. Dechert-Schmitt A. M. R. Ingle G. K. Mascitti V. Org. Lett. 2016;18:508–511. - PubMed
- Liu N. -W. Liang S. Manolikakes G. Adv. Synth. Catal. 2017;359:1308–1319.
- Chen Y. Murray P. R. Davies A. T. Willis M. C. J. Am. Chem. Soc. 2018;140:8781–8787. - PubMed
- Zhang F. Zheng D. Lai L. Cheng J. Sun J. Wu J. Org. Lett. 2018;20:1167–1170. - PubMed
- Marset X. Torregrosa-Crespo J. Martínez-Espinosa R. M. Guillena G. Ramón D. J. Green Chem. 2019;21:4127–4132.
- Wang X. Yang M. Kuang Y. Liu J.-B. Fan X. Wu J. Chem. Commun. 2020;56:3437–3440. - PubMed
- Blum S. P. Karakaya T. Schollmeyer D. Klapars A. Waldvogel S. R. Angew. Chem., Int. Ed. 2021;60:5056–5062. - PMC - PubMed
-
For examples of stepwise and one-pot approaches, see:
- Shavnya A. Coffey S. B. Hesp K. D. Ross S. C. Tsai A. S. Org. Lett. 2016;18:5848–5851. - PubMed
- Jiang Y.-Y. Wang Q.-Q. Liang S. Hu L.-M. Little R. D. Zeng C.-C. J. Org. Chem. 2016;81:4713–4719. - PubMed
- Kim D.-K. Um H.-S. Park H. Kim S. Choi J. Lee C. Chem. Sci. 2020;11:13071–13078. - PMC - PubMed
-
For examples of other C–S bond forming approaches to sulfonamides, see:
- Hell S. M. Meyer C. F. Laudadio G. Misale A. Willis M. C. Noël T. Trabanco A. A. Gouverneur V. J. Am. Chem. Soc. 2020;142:720–725. - PubMed
- Davies T. Q. Tilby M. J. Skolc D. Hall A. Willis M. C. Org. Lett. 2020;22:9495–9499. - PMC - PubMed
-
For examples of intermolecular tricomponent syntheses of aliphatic N-aminosulfonamides, see:
- Li Y. Zheng D. Li Z. Wu J. Org. Chem. Front. 2016;3:574–578.
- Zhou K. Xia H. Wu J. Org. Chem. Front. 2016;3:865–869.
-
For other recent approaches to sulfonamides that do not involve C–S bond formation, see:
- Hayashi E. Yamaguchi Y. Kita Y. Kamata K. Hara M. Chem. Commun. 2020;56:2095–2098. - PubMed
- Laudadio G. Barmpoutsis E. Schotten C. Struik L. Govaerts S. Browne D. L. Noël T. J. Am. Chem. Soc. 2019;141:5664–5668. - PMC - PubMed
- Tota A. St John-Campbell S. Briggs E. L. Estevez G. O. Afonso M. Degennaro L. Luisi R. Bull J. A. Org. Lett. 2018;20:2599–2602. - PubMed
-
-
- Bioactive Carboxylic Compound Classes: Pharmaceuticals and Agrochemicals, ed. C. Lamberth and J. Dinges, Wiley, 2016
- Iglesias J. Martínez-Salazar I. Maireles-Torres P. Martin Alonso D. Mariscal R. López Granados M. Chem. Soc. Rev. 2020;49:5704–5771. - PubMed
- Sinha J. Podgórski M. Huang S. Bowman C. N. Chem. Commun. 2018;54:3034–3037. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

