Ni(I)-Catalyzed β,δ-Vinylarylation of γ,δ-Alkenyl α-Cyanocarboxylic Esters via Contraction of Transient Nickellacycles
- PMID: 34084651
- PMCID: PMC8171255
Ni(I)-Catalyzed β,δ-Vinylarylation of γ,δ-Alkenyl α-Cyanocarboxylic Esters via Contraction of Transient Nickellacycles
Abstract
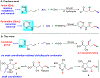
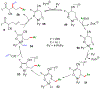
We disclose a transmetalation-initiated Ni(I)-catalyzed regioselective β,δ-vinylarylation of γ,δ-alkenyl α-cyanocarboxylic esters with vinyl triflates and arylzinc reagents. This reaction proceeds via contraction of six-membered nickellacycles to five-membered nickellacycles to form carbon-carbon bonds at the nonclassical homovicinal sites, and it provides expeditious access to a wide range of complex aliphatic α-cyanoesters, α-cyanocarboxylic acids, dicarboxylic acids, dicarboxylic acid monoamides, monocarboxylic acids, nitriles, and spirolactones. Control, deuterium labeling, and crossover experiments indicate that (i) the nickellacycle contraction occurs by β-H elimination, followed by hydronickellation on transiently formed alkenes, and (ii) the Ni species are stabilized as Ni-enolates.
Keywords: metallacycle contraction; nickel enolates; nickel(I)-catalyzed; regioselective; transmetalation; β,δ-vinylarylation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
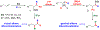
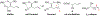

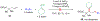
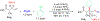

References
-
-
For reviews, see:
- Giri R; Kc S Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem 2018, 83, 3013–3022. - PubMed
- Dhungana RK; Kc S; Basnet P; Giri R Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec 2018, 18, 1314–1340. - PubMed
- Derosa J; Tran V; van der Puyl VA; Engle KM Carbon—Carbon π-Bonds as Conjunctive Reagents in Cross-Coupling. Aldrichimica Acta 2018, 51, 21–32.
-
-
-
For dicarbofunctionalization of activated alkenes, see:
- Qin T; Cornella J; Li C; Malins LR; Edwards JT; Kawamura S; Maxwell BD; Eastgate MD; Baran PS A General Alkyl-Alkyl Cross-Coupling Enabled by Redox-Active Esters and Alkylzinc Reagents. Science 2016, 352, 801–805. - PMC - PubMed
- Guo H-C; Ma J-A Catalytic Asymmetric Tandem Transformations Triggered by Conjugate Additions. Angew. Chem. Int. Ed 2006, 45, 354–366. - PubMed
- Zhang L; Lovinger GJ; Edelstein EK; Szymaniak AA; Chierchia MP; Morken JP Catalytic Conjunctive Cross-Coupling Enabled by Metal-Induced Metallate Rearrangement. Science 2016, 351, 70–74. - PMC - PubMed
-
-
-
For alkene dicarbofunctionalization via carbometalation, see:
- Nakamura M; Hirai A; Nakamura E Iron-Catalyzed Olefin Carbometalation. J. Am. Chem. Soc 2000, 122, 978–979.
-
-
- García-Domínguez A; Li Z; Nevado C Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc 2017, 139, 6835–6838. - PubMed
- Terao J; Saito K; Nii S; Kambe N; Sonoda N Regioselective Double Alkylation of Styrenes with Alkyl Halides Using a Titanocene Catalyst. J. Am. Chem. Soc 1998, 120, 11822–11823.
- Liao L; Jana R; Urkalan KB; Sigman MS A Palladium-Catalyzed Three-Component Cross-Coupling of Conjugated Dienes or Terminal Alkenes with Vinyl Triflates and Boronic Acids. J. Am. Chem. Soc 2011, 133, 5784–5787. - PMC - PubMed
- Wu X; Lin H-C; Li M-L; Li L-L; Han Z-Y; Gong L-Z Enantioselective 1,2-Difunctionalization of Dienes Enabled by Chiral Palladium Complex-Catalyzed Cascade Arylation/Allylic Alkylation Reaction. J. Am. Chem. Soc 2015, 137, 13476–13479. - PubMed
- Terao J; Nii S; Chowdhury FA; Nakamura A; Kambe N Nickel-Catalyzed Regioselective Three Component Coupling Reaction of Alkyl Halides, Butadienes, and ArM (M = MgX, ZnX). Adv. Synth. Catal 2004, 346, 905–908.
- Mizutani K; Shinokubo H; Oshima K Cobalt-Catalyzed Three-Component Coupling Reaction of Alkyl Halides, 1,3-Dienes, and Trimethylsilylmethylmagnesium Chloride. Org. Lett 2003, 5, 3959–3961. - PubMed
- Urkalan KB; Sigman MS Palladium-Catalyzed Oxidative Intermolecular Difunctionalization of Terminal Alkenes with Organostannanes and Molecular Oxygen. Angew. Chem., Int. Ed 2009, 48, 3146–3149. - PMC - PubMed
- Kuang Z; Yang K; Song Q Pd-Catalyzed Regioselective 1,2-Difunctionalization of Vinylarenes with Alkenyl Triflates and Aryl Boronic Acids at Ambient Temperature. Org. Lett 2017, 19, 2702–2705. - PubMed
- Kc S; Dhungana RK; Shrestha B; Thapa S; Khanal N; Basnet P; Lebrun RW; Giri R Ni-Catalyzed Regioselective Alkylarylation of Vinylarenes via C(sp3)–C(sp3)/C(sp3)–C(sp2) Bond Formation and Mechanistic Studies. J. Am. Chem. Soc 2018, 140, 9801–9805. - PubMed
- Gao P; Chen L-A; Brown MK Nickel-Catalyzed Stereoselective Diarylation of Alkenylarenes. J. Am. Chem. Soc 2018, 140, 10653–10657. - PMC - PubMed
- Anthony D; Lin Q; Baudet J; Diao T Nickel-Catalyzed Asymmetric Reductive Diarylation of Vinylarenes. Angew. Chem., Int. Ed 2019, 58, 3198–3202. - PMC - PubMed
- Klauck FJR; Yoon H; James MJ; Lautens M; Glorius F Visible-Light-Mediated Deaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal. 2019, 9, 236–241.
- Yong X; Han Y-F; Li Y; Song R-J; Li J-H Alkylarylation of Styrenes via Direct C(sp3)–Br/C(sp2)–H Functionalization Mediated by Photoredox and Copper Cooperative Catalysis. Chem. Commun 2018, 54, 12816–12819. - PubMed
- Ouyang X-H; Hu M; Song R-J; Li J-H Oxidative Three-Component 1,2-Alkylarylation of Alkenes with Alkyl Nitriles and N-Heteroarenes. Chem. Commun 2018, 54, 12345–12348. - PubMed
- Ouyang X-H; Cheng J; Li J-H 1,2-Diarylation of Alkenes with Aryldiazonium Salts and Arenes Enabled by Visible Light Photoredox Catalysis. Chem. Commun 2018, 54, 8745–8748. - PubMed
- Ouyang X-H; Li Y; Song R-J; Hu M; Luo S; Li J-H Intermolecular Dialkylation of Alkenes with Two Distinct C(sp3)-H Bonds Enabled by Synergistic Photoredox Catalysis and Iron Catalysis. Sci. Adv 2019, 5, No. eaav9839. - PMC - PubMed
-
- Saini V; Sigman MS Palladium-Catalyzed 1,1-Difunctionalization of Ethylene. J. Am. Chem. Soc 2012, 134, 11372–11375. - PMC - PubMed
- Werner EW; Urkalan KB; Sigman MS PdII-Catalyzed Oxidative 1,1-Diarylation of Terminal Olefins. Org. Lett 2010, 12, 2848–2851. - PMC - PubMed
- Saini V; Liao L; Wang Q; Jana R; Sigman MS Pd(0)-Catalyzed 1,1-Diarylation of Ethylene and Allylic Carbonates. Org. Lett 2013, 15, 5008–5011. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
