Lung volume reduction in emphysema: a pragmatic prospective cohort study
- PMID: 34084783
- PMCID: PMC8165372
- DOI: 10.1183/23120541.00877-2020
Lung volume reduction in emphysema: a pragmatic prospective cohort study
Erratum in
-
Erratum: "Lung volume reduction in emphysema: a pragmatic prospective cohort study". Christophe Dooms, Astrid Blondeel, Laurens J. Ceulemans, Johan Coolen, Stephanie Everaerts, Heleen Demeyer, Thierry Troosters Geert Verleden, Dirk Van Raemdonck and Wim Janssens. ERJ Open Res 2021; 7: 00877-2020.ERJ Open Res. 2021 Jun 21;7(2):50877-2020. doi: 10.1183/23120541.50877-2020. eCollection 2021 Apr. ERJ Open Res. 2021. PMID: 34164553 Free PMC article.
Abstract
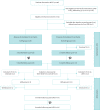
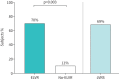
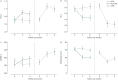
Limited guidance exists for the implementation of lung volume reduction interventions in routine clinical care. We designed a pragmatic study to evaluate a strategy including endoscopic lung volume reduction (ELVR) and lung volume reduction surgery (LVRS) in heterogeneous emphysema. This prospective monocentre cohort study evaluated ELVR versus no-ELVR, followed by a cohort study evaluating LVRS. Primary outcome was the proportion of subjects with a forced expiratory volume in 1 s (FEV1) improvement of ⩾100 mL at 3-month follow-up. Changes in FEV1, residual volume (RV), 6-min walk distance (6MWD) and quality of life (St George's Respiratory Questionnaire (SGRQ)) were evaluated at 6-month follow-up. Hospital stay and treatment-related serious adverse events were monitored. From 106 subjects screened, 38 subjects were enrolled comparing ELVR (n=20) with no-ELVR (n=18). After 6 months' follow-up, eligible patients were referred for LVRS (n=16) with another 6-month follow-up. At 3-month follow-up, 70% of ELVR compared to 11% of no-ELVR (p<0.001) and 69% of LVRS had an FEV1 improvement of ⩾100 mL. Between-group differences (mean±sem) for ELVR versus no-ELVR at 6-month follow-up were FEV1 +0.21±0.05 L; RV -0.95±0.21 L; 6MWD 58±17 m and SGRQ -18±5 points. At 6-month follow-up, within-group differences (mean±sem) for LVRS showed FEV1 +0.27±0.06 L; RV -1.49±0.22 L and 6MWD +75±18 m. Serious adverse events in 81% versus 45% of subjects (p=0.04) and a median hospital stay of 15 versus 5 days (p<0.001) were observed for LVRS versus ELVR, respectively. This pragmatic prospective cohort study supports a clinical approach with ELVR as a less invasive first option and LVRS as powerful alternative in severe heterogeneous emphysema.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: C. Dooms has nothing to disclose. Conflict of interest: A. Blondeel has nothing to disclose. Conflict of interest: L.J. Ceulemans has nothing to disclose. Conflict of interest: J. Coolen has nothing to disclose. Conflict of interest: S. Everaerts has nothing to disclose. Conflict of interest: H. Demeyer has nothing to disclose. Conflict of interest: T. Troosters has nothing to disclose. Conflict of interest: G. Verleden has nothing to disclose. Conflict of interest: D. Van Raemdonck has nothing to disclose. Conflict of interest: W. Janssens reports grants from PulmonX (for the endobronchial valves). He receives grants from AstraZeneca and Chiesi, outside the submitted work. W. Janssens is co-founder of ArtiQ, a KU Leuven spin-off company in respiratory diseases.
Figures
Comment in
-
Lung volume reduction in real clinical practice.ERJ Open Res. 2021 Jun 7;7(2):00258-2021. doi: 10.1183/23120541.00258-2021. eCollection 2021 Apr. ERJ Open Res. 2021. PMID: 34109245 Free PMC article.
References
-
- Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006; 82: 431–443. doi: 10.1016/j.athoracsur.2006.05.069 - DOI - PubMed
LinkOut - more resources
Full Text Sources
