Measurement batch differences and between-batch conversion of Alzheimer's disease cerebrospinal fluid biomarker values
- PMID: 34084888
- PMCID: PMC8144935
- DOI: 10.1002/dad2.12194
Measurement batch differences and between-batch conversion of Alzheimer's disease cerebrospinal fluid biomarker values
Abstract
Introduction: Batch differences in cerebrospinal fluid (CSF) biomarker measurement can introduce bias into analyses for Alzheimer's disease studies. We evaluated and adjusted for batch differences using statistical methods.
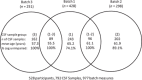
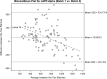
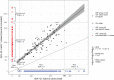
Methods: A total of 792 CSF samples from 528 participants were assayed in three batches for 12 biomarkers and 3 biomarker ratios. Batch differences were assessed using Bland-Altman plot, paired t test, Pitman-Morgan test, and linear regression. Generalized linear models were applied to convert CSF values between batches.
Results: We found statistically significant batch differences for all biomarkers and ratios, except that neurofilament light was comparable between batches 1 and 2. The conversion models generally had high R 2 except for converting P-tau between batches 1 and 3.
Discussion: Between-batch conversion allows harmonized CSF values to be used in the same analysis. Such method may be applied to adjust for other sources of variability in measuring CSF or other types of biomarkers.
Keywords: Alzheimer's disease; batch difference; biomarker; cerebrospinal fluid; conversion; generalized linear model.
© 2021 The Authors. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring published by Wiley Periodicals, LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Cynthia Carlsson receives grant support from NIH/Lilly, NIH, Veterans Affairs, and Bader Philanthropies. Dr. Sterling Johnson previously served on the advisory board for Roche Diagnostics. Dr. Sanjay Asthana serves as a site PI for pharmaceutical trials funded by Merck Pharmaceuticals, Lundbeck, NIH/UCSD, EISAI, and Genentech Inc. Dr. Richard Chappell serves on data safety monitoring boards for Axsome Pharmaceuticals and TGR Pharmaceuticals and has recently had a speaking engagement at Merck, Inc. Dr. Henrik Zetterberg has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Dr. Kaj Blennow has served as a consultant or on advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis, and Roche Diagnostics, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program.
Figures
References
-
- Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta‐analysis. Lancet Neurol. 2016;15:673‐684. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
