Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part II: treatment indications and complications
- PMID: 34085131
- PMCID: PMC8175681
- DOI: 10.1186/s13244-021-01001-w
Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging-Part II: treatment indications and complications
Abstract
Therapy of uveal melanoma aims to preserve the eye and its function and to avoid metastatic dissemination. The treatment choice is difficult and must keep into account several factors; the therapeutic strategy of uveal melanoma should therefore be personalized, sometimes requiring to combine different treatment techniques. Nowadays globe-sparing radiotherapy techniques are often preferred to enucleation. Plaque brachytherapy, the most commonly used eye-preserving therapy, is suitable for small- and medium-sized uveal melanomas. Proton beam radiotherapy is indicated for tumours with noticeable size, challenging shape and location, but is more expensive and less available than brachytherapy. Enucleation is currently restricted to advanced tumours, uveal melanomas with orbital or optic nerve involvement, blind and painful eyes because of treatment-related complications (neovascular glaucoma, chronic inflammatory processes). The effect of proton beam therapy on neoplastic tissue is related to direct cytotoxic action of the radiations, impairment of neoplastic vascular supply and immunologic response. Complications after radiotherapy are frequent and numerous and mainly related to tumour thickness, radiation dose and distance between the tumour and optic nerve. The purpose of this pictorial review is to provide the radiologists with awareness about diagnostic methods and therapeutic options of uveal melanoma. In the present second section, we discuss the therapeutic management of uveal melanoma, describing the main ocular-conserving radiotherapic techniques. We subsequently present an overview of the effects of radiations on neoplastic tissue. Lastly, we review ocular complications following radiotherapy that should be evaluated by radiologists during follow-up MRI examinations.
Keywords: Brachytherapy; Eye; Magnetic resonance imaging; Melanoma; Proton therapy.
Conflict of interest statement
The authors declare no competing interests in the manuscript.
Figures


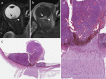
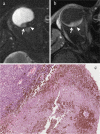
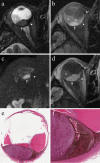


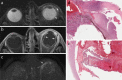



References
Publication types
LinkOut - more resources
Full Text Sources

