Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells
- PMID: 34085316
- PMCID: PMC8365755
- DOI: 10.1111/jpi.12747
Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells
Abstract
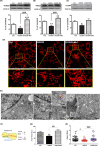
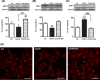
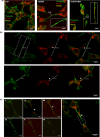
Mitochondrial dysfunction is considered one of the hallmarks of ischemia/reperfusion injury. Mitochondria are plastic organelles that undergo continuous biogenesis, fusion, and fission. They can be transferred between cells through tunneling nanotubes (TNTs), dynamic structures that allow the exchange of proteins, soluble molecules, and organelles. Maintaining mitochondrial dynamics is crucial to cell function and survival. The present study aimed to assess the effects of melatonin on mitochondrial dynamics, TNT formation, and mitochondria transfer in HT22 cells exposed to oxygen/glucose deprivation followed by reoxygenation (OGD/R). The results showed that melatonin treatment during the reoxygenation phase reduced mitochondrial reactive oxygen species (ROS) production, improved cell viability, and increased the expression of PGC1α and SIRT3. Melatonin also preserved the expression of the membrane translocase proteins TOM20 and TIM23, and of the matrix protein HSP60, which are involved in mitochondrial biogenesis. Moreover, it promoted mitochondrial fusion and enhanced the expression of MFN2 and OPA1. Remarkably, melatonin also fostered mitochondrial transfer between injured HT22 cells through TNT connections. These results provide new insights into the effect of melatonin on mitochondrial network reshaping and cell survival. Fostering TNTs formation represents a novel mechanism mediating the protective effect of melatonin in ischemia/reperfusion injury.
Keywords: HT22; melatonin; mitochondrial network; oxygen-glucose deprivation; tunneling nanotubes.
© 2021 The Authors. Journal of Pineal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors of this paper declare that they do not have any conflict of interest.
Figures



References
-
- Kim MJ, Cho JH, Cho JH, et al. Impact of hyperthermia before and during ischemia‐reperfusion on neuronal damage and gliosis in the gerbil hippocampus induced by transient cerebral ischemia. J Neurol Sci. 2015;348(1–2):101‐110. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

