Outcomes of COVID-19 in Patients With Cancer: Report From the National COVID Cohort Collaborative (N3C)
- PMID: 34085538
- PMCID: PMC8260918
- DOI: 10.1200/JCO.21.01074
Outcomes of COVID-19 in Patients With Cancer: Report From the National COVID Cohort Collaborative (N3C)
Abstract
Purpose: Variation in risk of adverse clinical outcomes in patients with cancer and COVID-19 has been reported from relatively small cohorts. The NCATS' National COVID Cohort Collaborative (N3C) is a centralized data resource representing the largest multicenter cohort of COVID-19 cases and controls nationwide. We aimed to construct and characterize the cancer cohort within N3C and identify risk factors for all-cause mortality from COVID-19.
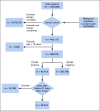
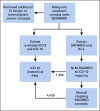
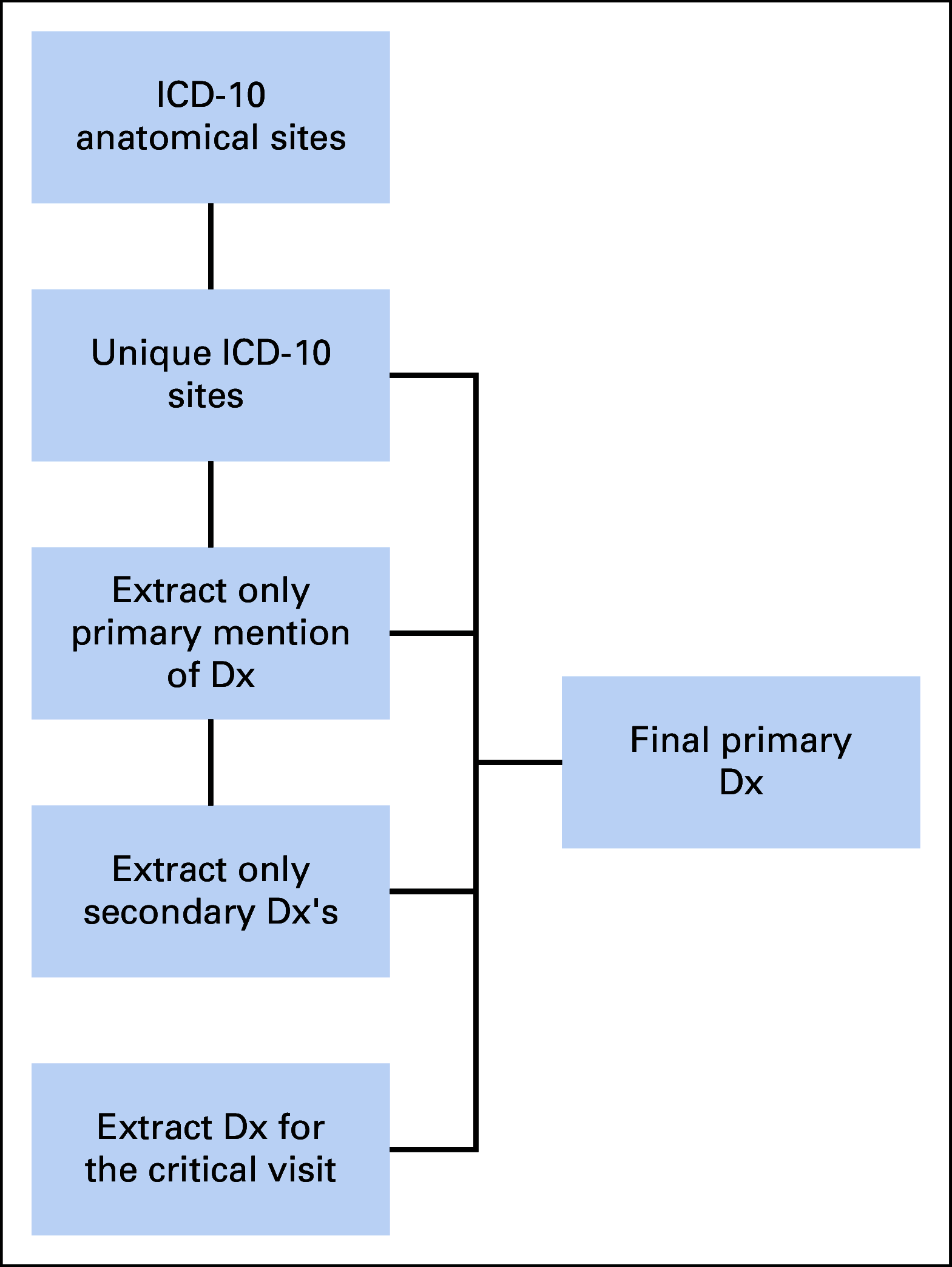
Methods: We used 4,382,085 patients from 50 US medical centers to construct a cohort of patients with cancer. We restricted analyses to adults ≥ 18 years old with a COVID-19-positive or COVID-19-negative diagnosis between January 1, 2020, and March 25, 2021. We followed N3C selection of an index encounter per patient for analyses. All analyses were performed in the N3C Data Enclave Palantir platform.
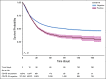
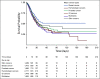
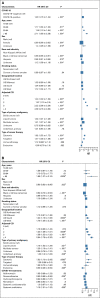
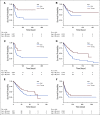
Results: A total of 398,579 adult patients with cancer were identified from the N3C cohort; 63,413 (15.9%) were COVID-19-positive. Most common represented cancers were skin (13.8%), breast (13.7%), prostate (10.6%), hematologic (10.5%), and GI cancers (10%). COVID-19 positivity was significantly associated with increased risk of all-cause mortality (hazard ratio, 1.20; 95% CI, 1.15 to 1.24). Among COVID-19-positive patients, age ≥ 65 years, male gender, Southern or Western US residence, an adjusted Charlson Comorbidity Index score ≥ 4, hematologic malignancy, multitumor sites, and recent cytotoxic therapy were associated with increased risk of all-cause mortality. Patients who received recent immunotherapies or targeted therapies did not have higher risk of overall mortality.
Conclusion: Using N3C, we assembled the largest nationally representative cohort of patients with cancer and COVID-19 to date. We identified demographic and clinical factors associated with increased all-cause mortality in patients with cancer. Full characterization of the cohort will provide further insights into the effects of COVID-19 on cancer outcomes and the ability to continue specific cancer treatments.
Conflict of interest statement
Figures
Comment in
-
Reply to K. Takada et al.J Clin Oncol. 2021 Dec 10;39(35):3997-3998. doi: 10.1200/JCO.21.02150. Epub 2021 Oct 8. J Clin Oncol. 2021. PMID: 34623872 Free PMC article. No abstract available.
-
Reasons to Consider the COVID-19 Vaccination Status of Patients With Cancer When Analyzing Their COVID-19 Outcomes.J Clin Oncol. 2021 Dec 10;39(35):3996. doi: 10.1200/JCO.21.01561. Epub 2021 Oct 8. J Clin Oncol. 2021. PMID: 34623886 Free PMC article. No abstract available.
References
-
- Lima NT, Buss PM, Paes-Sousa R. COVID-19 pandemic: A health and humanitarian crisis. Cad Saude Publica. 2020;36:e00177020. - PubMed
-
- Lunski MJ, Burton J, Tawagi K, et al. Multivariate mortality analyses in COVID-19: Comparing patients with cancer and patients without cancer in Louisiana Cancer 127266–2742021 - PubMed
Publication types
MeSH terms
Grants and funding
- U54 GM104938/GM/NIGMS NIH HHS/United States
- UL1 TR002649/TR/NCATS NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- UL1 TR001433/TR/NCATS NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- UL1 TR001439/TR/NCATS NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UL1 TR001445/TR/NCATS NIH HHS/United States
- UL1 TR003096/TR/NCATS NIH HHS/United States
- UL1 TR002537/TR/NCATS NIH HHS/United States
- UL1 TR001412/TR/NCATS NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- UL1 TR002529/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- UL1 TR002494/TR/NCATS NIH HHS/United States
- UL1 TR002736/TR/NCATS NIH HHS/United States
- U54 GM115516/GM/NIGMS NIH HHS/United States
- UL1 TR002369/TR/NCATS NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- UL1 TR002001/TR/NCATS NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- U54 GM115458/GM/NIGMS NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR001453/TR/NCATS NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- UL1 TR003015/TR/NCATS NIH HHS/United States
- UL1 TR002733/TR/NCATS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- P30 CA012197/CA/NCI NIH HHS/United States
- U24 TR002306/TR/NCATS NIH HHS/United States
- UL1 TR002003/TR/NCATS NIH HHS/United States
- UL1 TR001876/TR/NCATS NIH HHS/United States
- UL1 TR001436/TR/NCATS NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR002553/TR/NCATS NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- U54 GM104941/GM/NIGMS NIH HHS/United States
- UL1 TR002014/TR/NCATS NIH HHS/United States
- UL1 TR002550/TR/NCATS NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- UL1 TR001855/TR/NCATS NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- UL1 TR003017/TR/NCATS NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR002645/TR/NCATS NIH HHS/United States
- UL1 TR001450/TR/NCATS NIH HHS/United States
- UL1 TR002366/TR/NCATS NIH HHS/United States
- U54 GM115428/GM/NIGMS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- UL1 TR002377/TR/NCATS NIH HHS/United States
- U54 GM115677/GM/NIGMS NIH HHS/United States
- UL1 TR002544/TR/NCATS NIH HHS/United States
- UL1 TR003098/TR/NCATS NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

