Clinically Meaningful Improvements in Early Morning and Late Afternoon/Evening Functional Impairment in Children with ADHD Treated with Delayed-Release and Extended-Release Methylphenidate
- PMID: 34085581
- PMCID: PMC8785267
- DOI: 10.1177/10870547211020073
Clinically Meaningful Improvements in Early Morning and Late Afternoon/Evening Functional Impairment in Children with ADHD Treated with Delayed-Release and Extended-Release Methylphenidate
Abstract
Objective: The Before School Functioning Questionnaire and Parent Rating of Evening and Morning Behavior-Revised assess early morning (BSFQ, PREMB-R AM subscale) and late afternoon/evening (PREMB-R PM subscale) functional impairment in children with ADHD. Clinically meaningful improvements were identified and applied to a trial of delayed-release and extended-release methylphenidate (DR/ER-MPH) in children with ADHD (NCT02520388) to determine if the statistically-determined improvements in functional impairment were also clinically meaningful.
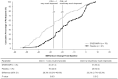
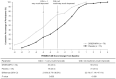
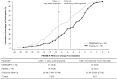
Method: Clinically meaningful improvements in BSFQ/PREMB-R were established post hoc by receiver operating characteristics curves, using anchors of Clinical Global Impression-Improvement (CGI-I) = 1 and CGI-I ≤ 2. Percentages of participants achieving these thresholds were calculated.
Results: Thresholds for CGI-I = 1/CGI-I ≤ 2, respectively, were 27/20 (BSFQ), 5/3 (PREMB-R AM), and 9/5 (PREMB-R PM)-point decreases. More children achieved clinically meaningful improvements with DR/ER-MPH versus placebo (all p < .05).
Conclusion: DR/ER-MPH increased proportions of children achieving clinically meaningful improvements in BSFQ and PREMB-R.
Keywords: ADHD; BSFQ; PREMB-R; functional impairment; methylphenidate.
Conflict of interest statement
Figures

References
-
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. 10.1176/appi.books.9780890425596 - DOI
-
- Brams M., Muniz R., Childress A., Giblin J., Mao A., Turnbow J., Borrello M., McCague K., Lopez F. A., Silva R. (2008). A randomized, double-blind, crossover study of once-daily dexmethylphenidate in children with attention-deficit/hyperactivity disorder: Rapid onset of effect. CNS Drugs, 22(8), 693–704. 10.2165/00023210-200822080-00006 - DOI - PubMed
-
- Canadian ADHD Resource Alliance. (2020). Canadian ADHD practice guidelines (4.1 ed.). https://www.caddra.ca/wp-content/uploads/CADDRA-ADHD-Practice-Guidelines...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

