Pharmacometric analysis linking immunoglobulin exposure to clinical efficacy outcomes in chronic inflammatory demyelinating polyneuropathy
- PMID: 34085779
- PMCID: PMC8376132
- DOI: 10.1002/psp4.12647
Pharmacometric analysis linking immunoglobulin exposure to clinical efficacy outcomes in chronic inflammatory demyelinating polyneuropathy
Abstract
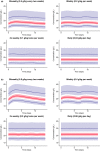
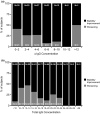
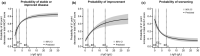
The two main objectives of this analysis were to (i) characterize the relationship between immunoglobulin (Ig) exposure and chronic inflammatory demyelinating polyneuropathy (CIDP) disease severity using data from 171 patients with CIDP who received either subcutaneous Ig (IgPro20; Hizentra® ) or placebo (PATH study), and to (ii) simulate and compare exposure coverage with various dosing approaches considering weekly dosing to be the reference dose. IgG pharmacokinetic (PK) parameters, including those from a previous population PK model, were used to predict individual IgG profile and exposure metrics. Treatment-related changes in Inflammatory Neuropathy Cause and Treatment (INCAT) scores were best described by a maximum effect (Emax ) model as a function of ΔIgG (total serum IgG at INCAT score assessment minus baseline IgG levels before intravenous Ig restabilization). Simulations indicate that flexible dosing from daily to biweekly (every other week) provide an exposure coverage equivalent to that of a weekly Ig dose.
© 2021 CSL Behring. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
V.B. is consultant to CSL Behring, Grifols, Pfizer, UCB, Bionevia, and ArgenX and has received research support from Baxalta (Shire), CSL Behring, Grifols, Bionevia UCB, and ArgenX. D.R.C. has acted as a consultant for Acetylon Pharmaceuticals Inc., Alnylam Pharmaceuticals, Annexon Biosciences, Akros Pharma, argenx BVBA, Biotest Pharmaceuticals, Inc., Boehringer Ingelheim, Cigna Health Management, Inc., CSL Behring, DP Clinical, Inc., Grifols S.A., Hansa Medical Inc., Karos Pharmaceuticals, Inc., Merrimack Pharmaceuticals, Inc., Neurocrine Biosciences, Novartis Corp., Octapharma AG, Pharnext SAS, Seattle Genetics, Inc., Sun Pharmaceuticals, and Syntimmune. D.R.C. has acted on a data safety monitoring board for Pfizer Inc., Ionis Pharmaceuticals, Axovant Sciences LTD., Ampio Pharmaceuticals, PledPharma, Momenta Pharma, and Sanofi. D.R.C. has a technology license with Acetylon Pharmaceuticals Inc., Akros Pharma, AstraZeneca Pharmaceuticals, LP, Calithera Biosciences, Genentech Inc, Karos Pharma, Neurocrine Biosciences, Merrimack Pharmaceuticals Inc., Seattle Genetics, Inc., and Shire Development, LLC. D.C.C. serves on the board of directors for The Peripheral Nerve Society and acts on the medical advisory board for GBS CIDP Foundation International. H.‐P.H. has acted on steering and data monitoring committees for Bayer Healthcare, Biogen, Celgene BMS, CSL Behring, GeNeuro, Merck, Novartis, Octapharma, Receptos, Roche, Sanofi Genzyme, TG Therapeutics, and VielaBio, and advisory boards for Alexion and Lundbeck. R.A.L. has received consultation fees and/or served on scientific advisory boards for CSL Behring, Axelacare, Pharnext, Biotest, Kedrion, NuFactor, Optioncare, and Grifols. J.‐P.L., M.T., T.Y., X.M., M.P., O.M., and B.L.D. are all employees of CSL Behring. I.S.J.M. reports grants from Talecris Talents Program/Perinoms study, grants from GBS CIDP Foundation International, grants from Princes Beatrix Foundation, grants from European Union 7th Framework Program, other from Steering committee member for various studies, outside the submitted work. He serves on the editorial board of the Journal of Peripheral Nervous system, is a member of the Inflammatory Neuropathy Consortium (INC), and member of the Peripheral Nerve Society. I.N.v.S. chairs a steering committee for CSL Behring and received departmental honoraria for serving on scientific advisory boards for CSL Behring. He received departmental research support from The Netherlands Organization for Scientific Research and from the Dutch Prinses Beatrix Fonds. All lecturing and consulting fees for INS were donated to the Stichting Klinische Neurologie, a local foundation that supports research in the field of neurological disorders. He is a member of the Scientific Board of the Kreuth III meeting on the optimal use of plasma‐derived medicinal products, especially coagulation factors and normal immunoglobulins organized under the auspices of the European Directorate for the Quality of Medicines & HealthCare (EDQM). G.S. has received funds from Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical Company Limited, and CSL Behring. All other authors declared no competing interests for this work.
Figures
References
-
- Bunschoten C, Jacobs BC, Van den Bergh PYK, Cornblath DR, van Doorn PA . Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019;18:784‐794. - PubMed
-
- Rajabally YA, Wong SL, Kearney DA. Immunoglobulin G level variations in treated chronic inflammatory demyelinating polyneuropathy: clues for future treatment regimens? J Neurol. 2013;260:2052‐2056. - PubMed
-
- Kuitwaard K, Fokkink W‐J, Brusse E, et al. Maintenance IV immunoglobulin treatment in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2017;22:425‐432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

