Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial
- PMID: 34086039
- PMCID: PMC8446818
- DOI: 10.1001/jamaoncol.2021.2301
Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients With Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial
Abstract
Importance: Administration of pembrolizumab plus concurrent chemoradiation therapy (cCRT) may provide treatment benefit to patients with locally advanced, stage III non-small cell lung cancer (NSCLC).
Objective: To evaluate treatment outcomes and safety of pembrolizumab plus cCRT in stage III NSCLC.
Design, setting, and participants: The phase 2, nonrandomized, 2-cohort, open-label KEYNOTE-799 study enrolled patients between November 5, 2018, and July 31, 2020, from 52 academic facilities and community-based institutions across 10 countries. As of October 28, 2020, median (range) follow-up was 18.5 (13.6-23.8) months in cohort A and 13.7 (2.9-23.5) months in cohort B. Of 301 patients screened, 216 eligible patients with previously untreated, unresectable, and pathologically/radiologically confirmed stage IIIA/IIIB/IIIC NSCLC with measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1) were enrolled.
Interventions: Patients in cohort A (squamous/nonsquamous) received 1 cycle (3 weeks) of carboplatin (area under the curve [AUC] 6 mg/mL/min), paclitaxel (200 mg/m2), and pembrolizumab (200 mg), followed by carboplatin (AUC 2 mg/mL/min) and paclitaxel (45 mg/m2) once weekly for 6 weeks and 2 cycles of pembrolizumab plus standard thoracic radiotherapy. Patients in cohort B (nonsquamous) received 3 cycles of cisplatin (75 mg/m2), pemetrexed (500 mg/m2), and pembrolizumab (200 mg) every 3 weeks and thoracic radiotherapy in cycles 2 and 3. Patients received 14 additional cycles of pembrolizumab.
Main outcomes and measures: Coprimary end points were objective response rate per RECIST v1.1 by blinded independent central review and incidence of grade 3 to 5 pneumonitis.
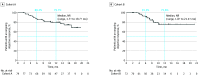
Results: A total of 112 patients received treatment in cohort A (76 men [67.9%]; median [range] age, 66.0 [46-90] years; 66 patients [58.9%] with programmed cell death ligand 1 [PD-L1] tumor proportion score ≥1%) and 102 patients received treatment in cohort B (62 men [60.8%]; median [range] age, 64.0 [35-81] years; 40 patients [39.2%] with PD-L1 tumor proportion score ≥1%). Objective response rate was 70.5% (79 of 112; 95% CI, 61.2%-78.8%) in cohort A and 70.6% (72 of 102; 95% CI, 60.7%-79.2%) in cohort B. Median duration of response was not reached, but 79.7% and 75.6%, respectively, had response duration of 12 months or longer. Grade 3 or higher pneumonitis occurred in 9 of 112 patients (8.0%) in cohort A and 7 of 102 (6.9%) in cohort B. Grade 3 to 5 treatment-related adverse events occurred in 72 of 112 (64.3%) and 51 of 102 (50.0%) patients, respectively.
Conclusions and relevance: The findings of this phase 2, nonrandomized, 2-cohort study suggest promising antitumor activity of pembrolizumab plus cCRT and manageable safety in patients with previously untreated, locally advanced, stage III NSCLC.
Conflict of interest statement
Figures

Comment in
-
Analysis of Outcomes With Addition of Immunotherapy to Chemoradiation Therapy for Non-Small Cell Lung Cancer-Reply.JAMA Oncol. 2022 Jan 1;8(1):168-169. doi: 10.1001/jamaoncol.2021.5611. JAMA Oncol. 2022. PMID: 34734971 No abstract available.
-
Analysis of Outcomes With Addition of Immunotherapy to Chemoradiation Therapy for Non-Small Cell Lung Cancer.JAMA Oncol. 2022 Jan 1;8(1):167-168. doi: 10.1001/jamaoncol.2021.5605. JAMA Oncol. 2022. PMID: 34734980 Free PMC article. No abstract available.
-
Analysis of Outcomes With Addition of Immunotherapy to Chemoradiation Therapy for Non-Small Cell Lung Cancer.JAMA Oncol. 2022 Jan 1;8(1):168. doi: 10.1001/jamaoncol.2021.5608. JAMA Oncol. 2022. PMID: 34734982 No abstract available.
References
-
- Imfinzi (durvalumab). Package insert. AstraZeneca AB; 2018.
LinkOut - more resources
Full Text Sources
Medical
Research Materials

