The cell biology of synapse formation
- PMID: 34086051
- PMCID: PMC8186004
- DOI: 10.1083/jcb.202103052
The cell biology of synapse formation
Abstract
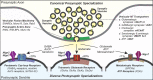
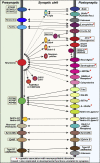
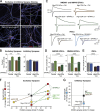
In a neural circuit, synapses transfer information rapidly between neurons and transform this information during transfer. The diverse computational properties of synapses are shaped by the interactions between pre- and postsynaptic neurons. How synapses are assembled to form a neural circuit, and how the specificity of synaptic connections is achieved, is largely unknown. Here, I posit that synaptic adhesion molecules (SAMs) organize synapse formation. Diverse SAMs collaborate to achieve the astounding specificity and plasticity of synapses, with each SAM contributing different facets. In orchestrating synapse assembly, SAMs likely act as signal transduction devices. Although many candidate SAMs are known, only a few SAMs appear to have a major impact on synapse formation. Thus, a limited set of collaborating SAMs likely suffices to account for synapse formation. Strikingly, several SAMs are genetically linked to neuropsychiatric disorders, suggesting that impairments in synapse assembly are instrumental in the pathogenesis of neuropsychiatric disorders.
© 2021 Südhof.
Figures


References
-
- Anderson, G.R., Aoto J., Tabuchi K., Földy F., Covy J., Yee A.X., Wu D., Lee S.-J., Chen L., Malenka R.C., and Südhof T.C.. 2015. β-Neurexins Control Neural Circuit Dynamics by Regulating Endocannabinoid Signaling at Excitatory Synapses. Cell. 162:593–606. 10.1016/j.cell.2015.06.056 - DOI - PMC - PubMed

