Pharmacokinetic and pharmacodynamic evaluation of nasal liposome and nanoparticle based rivastigmine formulations in acute and chronic models of Alzheimer's disease
- PMID: 34086100
- PMCID: PMC8298375
- DOI: 10.1007/s00210-021-02096-0
Pharmacokinetic and pharmacodynamic evaluation of nasal liposome and nanoparticle based rivastigmine formulations in acute and chronic models of Alzheimer's disease
Abstract
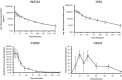
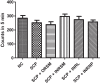
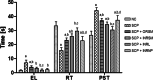
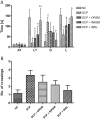
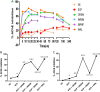
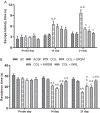
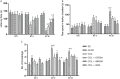
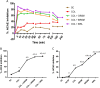
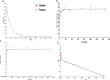
With the increasing aging population and progressive nature of the disease, Alzheimer's disease (AD) poses to be an oncoming epidemic with limited therapeutic strategies. It is characterized by memory loss, behavioral instability, impaired cognitive function, predominantly, cognitive inability manifested due to the accumulation of β-amyloid, with malfunctioned cholinergic system. Rivastigmine, a reversible dual cholinesterase inhibitor, is a more tolerable and widely used choice of drug for AD. However, rivastigmine being hydrophilic and undergoing the first-pass metabolism exhibits low CNS bioavailability. Nanoformulations including liposomes and PLGA nanoparticles can encapsulate hydrophilic drugs and deliver them efficiently to the brain. Besides, the nasal route is receiving considerable attention recently, due to its direct access to the brain. Therefore, the present study attempts to evaluate the pharmacokinetic and pharmacodynamic properties of nasal liposomal and PLGA nanoparticle formulations of rivastigmine in acute scopolamine-induced amnesia and chronic colchicine induced cognitive dysfunction animal models, and validate the best formulation by employing pharmacokinetic and pharmacodynamic (PK-PD) modeling. Nasal liposomal rivastigmine formulation showed the best pharmacokinetic features with rapid onset of action (Tmax = 5 min), higher Cmax (1489.5 ± 620.71), enhanced systemic bioavailability (F = 118.65 ± 23.54; AUC = 35,921.75 ± 9559.46), increased half-life (30.92 ± 8.38 min), and reduced clearance rate (Kel (1/min) = 0.0224 ± 0.006) compared to oral rivastigmine (Tmax = 15 min; Cmax = 56.29 ± 27.05; F = 4.39 ± 1.82; AUC = 1663.79 ± 813.54; t1/2 = 13.48 ± 5.79; Kel (1/min) = 0.0514 ± 0.023). Further, the liposomal formulation significantly rescued the memory deficit induced by scopolamine as well as colchicine superior to other formulations as assessed in Morris water maze and passive avoidance tasks. PK-PD modeling demonstrated a strong correlation between the pharmacokinetic parameters and acetylcholinesterase inhibition of liposomal formulation.
Keywords: Amnesia; Nanoformulation; Neurodegeneration; Nose to brain delivery; PK-PD modeling.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
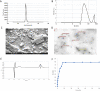
Figures


Similar articles
-
Intranasal administration of in silico designed Rivastigmine mucoadhesive nanoparticles ameliorates scopolamine-induced Alzheimer's symptoms in mice: Pharmacokinetic and pharmacodynamic evidences.Int J Pharm. 2025 May 30;677:125635. doi: 10.1016/j.ijpharm.2025.125635. Epub 2025 Apr 30. Int J Pharm. 2025. PMID: 40316192
-
Formulation and In-vivo Pharmacokinetic Consideration of Intranasal Microemulsion and Mucoadhesive Microemulsion of Rivastigmine for Brain Targeting.Pharm Res. 2018 Jan 2;35(1):8. doi: 10.1007/s11095-017-2279-z. Pharm Res. 2018. PMID: 29294189
-
Nose to brain microemulsion-based drug delivery system of rivastigmine: formulation and ex-vivo characterization.Drug Deliv. 2015;22(7):918-30. doi: 10.3109/10717544.2013.878857. Epub 2014 Jan 27. Drug Deliv. 2015. PMID: 24467601
-
Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease.Clin Ther. 1998 Jul-Aug;20(4):634-47. doi: 10.1016/s0149-2918(98)80127-6. Clin Ther. 1998. PMID: 9737824 Review.
-
The Revival of Scopolamine Reversal for the Assessment of Cognition-Enhancing Drugs.In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 17. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 17. PMID: 21204339 Free Books & Documents. Review.
Cited by
-
ADME/PK Insights of Crocetin: A Molecule Having an Unusual Chemical Structure with Druglike Features.ACS Omega. 2024 May 2;9(19):21494-21509. doi: 10.1021/acsomega.4c02116. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764638 Free PMC article.
-
Recent Advances in Therapeutics for the Treatment of Alzheimer's Disease.Molecules. 2024 Oct 30;29(21):5131. doi: 10.3390/molecules29215131. Molecules. 2024. PMID: 39519769 Free PMC article. Review.
-
Nanotherapeutic smart approaches for combating Alzheimer's disease and overcoming existing obstacles: A novel eco-friendly green approach.Toxicol Rep. 2025 Jan 27;14:101906. doi: 10.1016/j.toxrep.2025.101906. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 39926413 Free PMC article. Review.
-
Nanoparticle-based Gene Therapy for Neurodegenerative Disorders.Mini Rev Med Chem. 2024;24(19):1723-1745. doi: 10.2174/0113895575301011240407082559. Mini Rev Med Chem. 2024. PMID: 38676491 Review.
-
Drug Delivery Systems as a Strategy to Improve the Efficacy of FDA-Approved Alzheimer's Drugs.Pharmaceutics. 2022 Oct 26;14(11):2296. doi: 10.3390/pharmaceutics14112296. Pharmaceutics. 2022. PMID: 36365114 Free PMC article. Review.
References
-
- (2020) 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 16: 391-460 - PubMed
-
- Akbuǧa J, Bergişadi N. Effect of drug properties on physical and release characteristics of Eudragit microspheres prepared by spherical crystallization technique. Drug Dev Ind Pharm. 1997;23:407–411. doi: 10.3109/03639049709146145. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

