Directly observed therapy at opioid substitution facilities using sofosbuvir/velpatasvir results in excellent SVR12 rates in PWIDs at high risk for non-adherence to DAA therapy
- PMID: 34086708
- PMCID: PMC8177501
- DOI: 10.1371/journal.pone.0252274
Directly observed therapy at opioid substitution facilities using sofosbuvir/velpatasvir results in excellent SVR12 rates in PWIDs at high risk for non-adherence to DAA therapy
Abstract
Background & aims: We evaluated the effectiveness of sofosbuvir/velpatasvir (SOF/VEL) in difficult-to-treat PWIDs with presumed high risk for non-adherence to antiviral therapy using an innovative concept involving their opioid agonist therapy (OAT) facility.
Methods: N = 221 patients (m/f: 168/53; median age: 44.7 years (IQR 16.9); HCV-genotype 3: 45.2%; cirrhosis: 33.9%) treated with SOF/VEL were included. PWIDs at high risk for non-adherence to DAA therapy (n = 122) received HCV treatment alongside OAT under the supervision of medical staff ("directly observed therapy", DOT). These patients were compared to patients with presumed excellent drug compliance, who were treated in a "standard setting" (SS) of SOF/VEL prescription at a tertiary care center (n = 99).
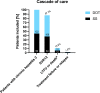
Results: DOT-patients (n = 122/221; 55.2%) were younger than SS-patients (median age: 41.3 vs. 53.0 years), all had psychiatric comorbidities and most had a poor socioeconomic status. 83/122 (68.0%) reported ongoing intravenous drug use. Within the DOT-group, SVR12 was achieved in 99.1% (95% CI: 95.0-100; n = 109/110) with one patient experiencing treatment failure, while n = 12/122 (9.8%) patients were excluded due to loss of follow-up (FU). 5 patients showed HCV reinfection after achieving SVR12. SS-patients achieved SVR in 96.6% (95% CI: 90.3-99.3%; n = 84/87) after exclusion of 10/99 (10.1%) patients who were lost to FU and 2 patients who died prior to SVR12 due to reasons not related to DAA therapy.
Conclusions: SOF/VEL given as DOT along with OAT in PWIDs at high risk of non-adherence to antiviral therapy including those with ongoing intravenous drug use resulted in excellent SVR rates similar to patients with presumed "excellent compliance" under standard drug intake.
Conflict of interest statement
C. Schmidbauer (schmidbauer.c@gmail.com): received travel support from Gilead, Abbvie and Gebro; and speaking honoraria from Abbvie. M. Schwarz (michael.schwarz@wienkav.at): received travel support from MSD, Sandoz, BMS and Abbvie; and speaking honoraria from BMS. A. Schütz (angelika.schuetz@suchthilfe.at): is employed by Suchthilfe Wien gGmbH and has no conflicts of interest. R. Schubert (raphael.schubert@outlook.at): is employed by Suchthilfe Wien gGmbH and received travel support from Gilead. C. Schwanke (cornelia.schwanke@suchthilfe.at): is employed by Suchthilfe Wien gGmbH and has no conflicts of interest. E. Gutic (enisa.gutic@extern.wienkav.at): received travel support from Gilead and Abbvie. R. Pirker (roxana.pirker@aon.at): no conflicts of interest. T. Lang (tobiaslang1904@gmail.com): no conflicts of interest. T. Reiberger (thomas.reiberger@meduniwien.ac.at): received grant support from Abbvie, Boehringer- Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Abbvie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fees from Abbvie, Bayer, Boehringer-Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer-Ingelheim, Gilead and Roche. H. Haltmayer (hans.haltmayer@suchthilfe.at): is employed by Suchthilfe Wien gGmbH and has no conflicts of interest. M. Gschwantler (michael.gschwantler@wienkav.at): received grants from Abbvie, Gilead, MSD; speaking honoraria from Abbvie, Gilead, MSD, Janssen, Roche, Intercept; consulting/advisory board fees from Abbvie, Gilead, MSD, Janssen, Roche, Intercept. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Directly observed therapy for HCV with glecaprevir/pibrentasvir alongside opioid substitution in people who inject drugs-First real world data from Austria.PLoS One. 2020 Mar 10;15(3):e0229239. doi: 10.1371/journal.pone.0229239. eCollection 2020. PLoS One. 2020. PMID: 32155165 Free PMC article.
-
Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3.J Hepatol. 2019 Jan;70(1):15-23. doi: 10.1016/j.jhep.2018.09.018. Epub 2018 Sep 26. J Hepatol. 2019. PMID: 30266283
-
Real-world effectiveness and safety of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir hepatitis C treatment in a single centre in Germany.PLoS One. 2019 Apr 4;14(4):e0214795. doi: 10.1371/journal.pone.0214795. eCollection 2019. PLoS One. 2019. PMID: 30946776 Free PMC article.
-
Treatment failure with DAA therapy: Importance of resistance.J Hepatol. 2021 Jun;74(6):1472-1482. doi: 10.1016/j.jhep.2021.03.004. Epub 2021 Mar 12. J Hepatol. 2021. PMID: 33716089 Review.
-
Sofosbuvir, velpatasvir and voxilaprevir combination for the treatment of hepatitis C.Expert Rev Gastroenterol Hepatol. 2017 Sep;11(9):789-795. doi: 10.1080/17474124.2017.1351295. Epub 2017 Jul 31. Expert Rev Gastroenterol Hepatol. 2017. PMID: 28673106 Review.
Cited by
-
Combining treatment for chronic hepatitis C with opioid agonist therapy is an effective microelimination strategy for people who inject drugs with high risk of non-adherence to direct-acting antiviral therapy.J Virus Erad. 2023 Mar 2;9(1):100319. doi: 10.1016/j.jve.2023.100319. eCollection 2023 Mar. J Virus Erad. 2023. PMID: 36970063 Free PMC article.
-
Out-of-Hospital Treatment of Hepatitis C Increases Retention in Care among People Who Inject Drugs and Homeless Persons: An Observational Study.J Clin Med. 2021 Oct 26;10(21):4955. doi: 10.3390/jcm10214955. J Clin Med. 2021. PMID: 34768474 Free PMC article.
-
ELIMINATE: a PCR record-based macroelimination project for systematic recall of HCV-RNA-positive persons in Austria.Wien Klin Wochenschr. 2024 May;136(9-10):278-288. doi: 10.1007/s00508-023-02275-4. Epub 2023 Sep 29. Wien Klin Wochenschr. 2024. PMID: 37773541 Free PMC article.
-
A systematic PCR record-based re-call of HCV-RNA-positive people enables re-linkage to care and HCV elimination in Austria - The ELIMINATE project.Liver Int. 2024 Dec;44(12):3151-3163. doi: 10.1111/liv.16076. Epub 2024 Oct 1. Liver Int. 2024. PMID: 39351692 Free PMC article.
-
Viral Hepatitis C New Microelimination Pathways Objective: Psychiatric Communities HCV Free.Life (Basel). 2022 Nov 13;12(11):1873. doi: 10.3390/life12111873. Life (Basel). 2022. PMID: 36431008 Free PMC article.
References
-
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030 [Internet]. 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

