Adherent and suspension baby hamster kidney cells have a different cytoskeleton and surface receptor repertoire
- PMID: 34086711
- PMCID: PMC8177424
- DOI: 10.1371/journal.pone.0246610
Adherent and suspension baby hamster kidney cells have a different cytoskeleton and surface receptor repertoire
Abstract
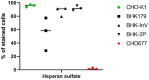
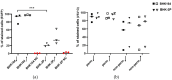
Animal cell culture, with single cells growing in suspension, ideally in a chemically defined environment, is a mainstay of biopharmaceutical production. The synthetic environment lacks exogenous growth factors and usually requires a time-consuming adaptation process to select cell clones that proliferate in suspension to high cell numbers. The molecular mechanisms that facilitate the adaptation and that take place inside the cell are largely unknown. Especially for cell lines that are used for virus antigen production such as baby hamster kidney (BHK) cells, the restriction of virus growth through the evolution of undesired cell characteristics is highly unwanted. The comparison between adherently growing BHK cells and suspension cells with different susceptibility to foot-and-mouth disease virus revealed differences in the expression of cellular receptors such as integrins and heparan sulfates, and in the organization of the actin cytoskeleton. Transcriptome analyses and growth kinetics demonstrated the diversity of BHK cell lines and confirmed the importance of well-characterized parental cell clones and mindful screening to make sure that essential cellular features do not get lost during adaptation.
Conflict of interest statement
The authors declare no conflict of interest. VD’s position was funded by a project grant from Merck Life Science. AZ is an employee of Merck Life Science. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Importance of Interaction between Integrin and Actin Cytoskeleton in Suspension Adaptation of CHO cells.Appl Biochem Biotechnol. 2016 Apr;178(7):1286-302. doi: 10.1007/s12010-015-1945-z. Epub 2015 Dec 17. Appl Biochem Biotechnol. 2016. PMID: 26679704 Free PMC article.
-
Identification of a novel cell culture adaptation site on the capsid of foot-and-mouth disease virus.J Gen Virol. 2015 Sep;96(9):2684-2692. doi: 10.1099/jgv.0.000222. Epub 2015 Jul 3. J Gen Virol. 2015. PMID: 26296881 Free PMC article.
-
Effects of two amino acid substitutions in the capsid proteins on the interaction of two cell-adapted PanAsia-1 strains of foot-and-mouth disease virus serotype O with heparan sulfate receptor.Virol J. 2014 Jul 24;11:132. doi: 10.1186/1743-422X-11-132. Virol J. 2014. PMID: 25056022 Free PMC article.
-
Single Amino Acid Substitutions Surrounding the Icosahedral Fivefold Symmetry Axis Are Critical for Alternative Receptor Usage of Foot-and-Mouth Disease Virus.Viruses. 2020 Oct 9;12(10):1147. doi: 10.3390/v12101147. Viruses. 2020. PMID: 33050303 Free PMC article.
-
Problems with BHK 21 cells.Dev Biol Stand. 1998;93:85-8. Dev Biol Stand. 1998. PMID: 9737382 Review.
Cited by
-
A Review of the Utility of Established Cell Lines for Isolation and Propagation of the Southern African Territories Serotypes of Foot-and-Mouth Disease Virus.Viruses. 2024 Dec 30;17(1):39. doi: 10.3390/v17010039. Viruses. 2024. PMID: 39861828 Free PMC article. Review.
-
Phytochemical Characterization, Antioxidant Activity, and Cytotoxicity of Methanolic Leaf Extract of Chlorophytum Comosum (Green Type) (Thunb.) Jacq.Molecules. 2022 Jan 24;27(3):762. doi: 10.3390/molecules27030762. Molecules. 2022. PMID: 35164026 Free PMC article.
-
Protein characterization of an Indonesian isolate of foot and mouth disease virus inactivated with formaldehyde and binary ethylenimine.Vet World. 2024 Aug;17(8):1836-1845. doi: 10.14202/vetworld.2024.1836-1845. Epub 2024 Aug 24. Vet World. 2024. PMID: 39328437 Free PMC article.
-
An In Vitro Study of Chitosan-Coated Bovine Pericardium as a Dural Substitute Candidate.J Funct Biomater. 2023 Sep 22;14(10):488. doi: 10.3390/jfb14100488. J Funct Biomater. 2023. PMID: 37888153 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

