CRISPR-targeted MAGT1 insertion restores XMEN patient hematopoietic stem cells and lymphocytes
- PMID: 34086870
- PMCID: PMC8718624
- DOI: 10.1182/blood.2021011192
CRISPR-targeted MAGT1 insertion restores XMEN patient hematopoietic stem cells and lymphocytes
Abstract
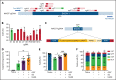
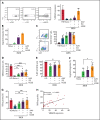
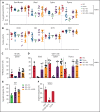
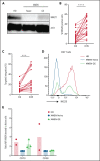
XMEN disease, defined as "X-linked MAGT1 deficiency with increased susceptibility to Epstein-Barr virus infection and N-linked glycosylation defect," is a recently described primary immunodeficiency marked by defective T cells and natural killer (NK) cells. Unfortunately, a potentially curative hematopoietic stem cell transplantation is associated with high mortality rates. We sought to develop an ex vivo targeted gene therapy approach for patients with XMEN using a CRISPR/Cas9 adeno-associated vector (AAV) to insert a therapeutic MAGT1 gene at the constitutive locus under the regulation of the endogenous promoter. Clinical translation of CRISPR/Cas9 AAV-targeted gene editing (GE) is hampered by low engraftable gene-edited hematopoietic stem and progenitor cells (HSPCs). Here, we optimized GE conditions by transient enhancement of homology-directed repair while suppressing AAV-associated DNA damage response to achieve highly efficient (>60%) genetic correction in engrafting XMEN HSPCs in transplanted mice. Restored MAGT1 glycosylation function in human NK and CD8+ T cells restored NK group 2 member D (NKG2D) expression and function in XMEN lymphocytes for potential treatment of infections, and it corrected HSPCs for long-term gene therapy, thus offering 2 efficient therapeutic options for XMEN poised for clinical translation.
© 2021 by The American Society of Hematology.
Figures


Comment in
-
Is this a cure for XMEN?Blood. 2021 Dec 30;138(26):2743-2744. doi: 10.1182/blood.2021012755. Blood. 2021. PMID: 34967867 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

