Different cardiovascular and pulmonary phenotypes for single- and double-knock-out mice deficient in BMP9 and BMP10
- PMID: 34086873
- PMCID: PMC9215199
- DOI: 10.1093/cvr/cvab187
Different cardiovascular and pulmonary phenotypes for single- and double-knock-out mice deficient in BMP9 and BMP10
Abstract
Aims: BMP9 and BMP10 mutations were recently identified in patients with pulmonary arterial hypertension, but their specific roles in the pathogenesis of the disease are still unclear. We aimed to study the roles of BMP9 and BMP10 in cardiovascular homeostasis and pulmonary hypertension using transgenic mouse models deficient in Bmp9 and/or Bmp10.
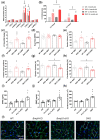
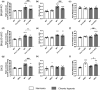
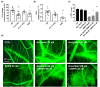
Methods and results: Single- and double-knockout mice for Bmp9 (constitutive) and/or Bmp10 (tamoxifen inducible) were generated. Single-knock-out (KO) mice developed no obvious age-dependent phenotype when compared with their wild-type littermates. However, combined deficiency in Bmp9 and Bmp10 led to vascular defects resulting in a decrease in peripheral vascular resistance and blood pressure and the progressive development of high-output heart failure and pulmonary hemosiderosis. RNAseq analysis of the lungs of the double-KO mice revealed differential expression of genes involved in inflammation and vascular homeostasis. We next challenged these mice to chronic hypoxia. After 3 weeks of hypoxic exposure, Bmp10-cKO mice showed an enlarged heart. However, although genetic deletion of Bmp9 in the single- and double-KO mice attenuated the muscularization of pulmonary arterioles induced by chronic hypoxia, we observed no differences in Bmp10-cKO mice. Consistent with these results, endothelin-1 levels were significantly reduced in Bmp9 deficient mice but not Bmp10-cKO mice. Furthermore, the effects of BMP9 on vasoconstriction were inhibited by bosentan, an endothelin receptor antagonist, in a chick chorioallantoic membrane assay.
Conclusions: Our data show redundant roles for BMP9 and BMP10 in cardiovascular homeostasis under normoxic conditions (only combined deletion of both Bmp9 and Bmp10 was associated with severe defects) but highlight specific roles under chronic hypoxic conditions. We obtained evidence that BMP9 contributes to chronic hypoxia-induced pulmonary vascular remodelling, whereas BMP10 plays a role in hypoxia-induced cardiac remodelling in mice.
Keywords: Bone morphogenetic proteins; High-output heart failure; Pulmonary hypertension; Pulmonary vascular remodelling; Vascular anomalies.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures




References
-
- Evans JD, Girerd B, Montani D, Wang XJ, Galie N, Austin ED, Elliott G, Asano K, Grunig E, Yan Y, Jing ZC, Manes A, Palazzini M, Wheeler LA, Nakayama I, Satoh T, Eichstaedt C, Hinderhofer K, Wolf M, Rosenzweig EB, Chung WK, Soubrier F, Simonneau G, Sitbon O, Graf S, Kaptoge S, Di Angelantonio E, Humbert M, Morrell NW.. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 2016;4:129–137. - PMC - PubMed
-
- David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S.. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007;109:1953–1961. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

