Exploring the Food and Drug Administration's review and approval of Entresto (sacubitril/valsartan)
- PMID: 34087050
- PMCID: PMC8177063
- DOI: 10.1002/prp2.794
Exploring the Food and Drug Administration's review and approval of Entresto (sacubitril/valsartan)
Abstract
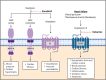
Federal regulatory agencies such as the United States Food and Drug Administration review pharmacological evidence to ensure the safety and efficacy of new and repurposed pharmaceuticals prior to market approval. The discussions, disagreements and procedural decisions contained within such reviews offer unique insight into a pharmaceutical's strengths, weaknesses and opportunities, yet are often overlooked as a significant source of pharmacological information for research and development. To highlight the value of such resources, we present a case study on Entresto, a first-in-class angiotensin receptor-neprilysin inhibitor for the treatment of heart failure with reduced ejection fraction, and explore the regulatory rationale underlying its market approval. Using information extracted from Entresto's online approval package at Drugs@FDA, we explore some of the procedural complexities underlying market approval of new pharmaceuticals, discuss the broad pharmacological implications contained within regulatory agency grey literature, and highlight opportunities for future therapeutic development.
Keywords: NT-proBNP; PARADIGM-HF; PARAGON-HF; pediatric heart failure; postmarketing requirements; sex differences.
© 2021 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
Mr. Herder reported being a member of the Patented Medicine Prices Review Board, Canada's national drug price regulator, and receiving honoraria from the Board for his service.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

