Roles of Fe-Histidine bonds in stability of hemoglobin: Recognition of protein flexibility by Q Sepharose
- PMID: 34087219
- PMCID: PMC8390899
- DOI: 10.1016/j.bpj.2021.05.014
Roles of Fe-Histidine bonds in stability of hemoglobin: Recognition of protein flexibility by Q Sepharose
Abstract
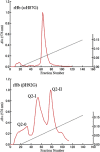
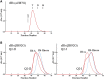
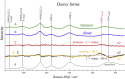
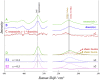
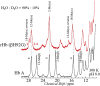
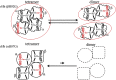
Using various mutants, we investigated to date the roles of the Fe-histidine (F8) bonds in cooperative O2 binding of human hemoglobin (Hb) and differences in roles between α- and β-subunits in the α2β2 tetramer. An Hb variant with a mutation in the heme cavity exhibited an unexpected feature. When the β mutant rHb (βH92G), in which the proximal histidine (His F8) of the β-subunit is replaced by glycine (Gly), was subjected to ion-exchange chromatography (Q Sepharose column) and eluted with an NaCl concentration gradient in the presence of imidazole, yielded two large peaks, whereas the corresponding α-mutant, rHb (αH87G), gave a single peak similar to Hb A. The β-mutant rHb proteins under each peak had identical isoelectric points according to isoelectric focusing electrophoresis. Proteins under each peak were further characterized by Sephadex G-75 gel filtration, far-UV CD, 1H NMR, and resonance Raman spectroscopy. We found that rHb (βH92G) exists as a mixture of αβ-dimers and α2β2 tetramers, and that hemes are released from β-subunits in a fraction of the dimers. An approximate amount of released hemes were estimated to be as large as 30% with Raman relative intensities. It is stressed that Q Sepharose columns can distinguish differences in structural flexibility of proteins having identical isoelectric points by altering the exit rates from the porous beads. Thus, the role of Fe-His (F8) bonds in stabilizing the Hb tetramer first described by Barrick et al. was confirmed in this study. In addition, it was found in this study that a specific Fe-His bond in the β-subunit minimizes globin structural flexibility.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Perutz M.F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu. Rev. Biochem. 1979;48:327–386. - PubMed
-
- Imai K. Cambridge University Press; Cambridge, UK: 1982. Allosteric Effects in Haemoglobin.
-
- Perutz M.F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970;228:726–739. - PubMed
-
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 1979;129:175–220. - PubMed
-
- Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

