Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic
- PMID: 34088556
- PMCID: PMC8107198
- DOI: 10.1016/j.amepre.2021.03.011
Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic
Abstract
Introduction: The COVID-19 pandemic has disrupted healthcare services, reducing opportunities to conduct routine hepatitis C virus antibody screening, clinical care, and treatment. Therefore, people living with undiagnosed hepatitis C virus during the pandemic may later become identified at more advanced stages of the disease, leading to higher morbidity and mortality rates. Further, unidentified hepatitis C virus-infected individuals may continue to unknowingly transmit the virus to others.
Methods: To assess the impact of the COVID-19 pandemic, data were evaluated from a large national reference clinical laboratory and from national estimates of dispensed prescriptions for hepatitis C virus treatment. Investigators estimated the average number of hepatitis C virus antibody tests, hepatitis C virus antibody-positive test results, and hepatitis C virus RNA-positive test results by month in January-July for 2018 and 2019, compared with the same months in 2020. To assess the impact of hepatitis C virus treatment, dispensed hepatitis C virus direct-acting antiretroviral medications were examined for the same time periods. Statistical analyses of trends were performed using negative binomial models.
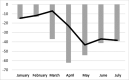
Results: Compared with the 2018 and 2019 months, hepatitis C virus antibody testing volume decreased 59% during April 2020 and rebounded to a 6% reduction in July 2020. The number of hepatitis C virus RNA-positive results fell by 62% in March 2020 and remained 39% below the baseline by July 2020. For hepatitis C virus treatment, prescriptions decreased 43% in May, 37% in June, and 38% in July relative to the corresponding months in 2018 and 2019.
Conclusions: During the COVID-19 pandemic, continued public health messaging, interventions and outreach programs to restore hepatitis C virus testing and treatment to prepandemic levels, and maintenance of public health efforts to eliminate hepatitis C infections remain important.
Copyright © 2021 American Journal of Preventive Medicine. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Centers for Disease for Control and Prevention; August 7, 2020. 2018 Surveillance report.https://www.cdc.gov/hepatitis/statistics/2018surveillance/index.htm Updated.
-
- Smith BD, Morgan RL, Beckett GA. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. [published correction appears in MMWR Recomm Rep. 2012;61(43):886] MMWR Recomm Rep. 2012;61(RR-4):1–32. www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm Updated. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

