Renin-Angiotensin System Blockers and the Risk of COVID-19-Related Mortality in Patients with Kidney Failure
- PMID: 34088718
- PMCID: PMC8425613
- DOI: 10.2215/CJN.18961220
Renin-Angiotensin System Blockers and the Risk of COVID-19-Related Mortality in Patients with Kidney Failure
Abstract
Background and objectives: There is concern about potential deleterious effects of angiotensin-converting enzyme inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) in patients with coronavirus disease 2019 (COVID-19). Patients with kidney failure, who often use ACEis/ARBs, are at higher risk of more severe COVID-19. However, there are no data available on the association of ACEi/ARB use with COVID-19 severity in this population.
Design, setting, participants, & measurements: From the European Renal Association COVID-19 database (ERACODA), we retrieved data on kidney transplant recipients and patients on dialysis who were affected by COVID-19, between February 1 and October 1, 2020, and had information on 28-day mortality. We used Cox proportional-hazards regression to calculate hazard ratios for the association between ACEi/ARB use and 28-day mortality risk. Additionally, we studied the association of discontinuation of these agents with 28-day mortality.
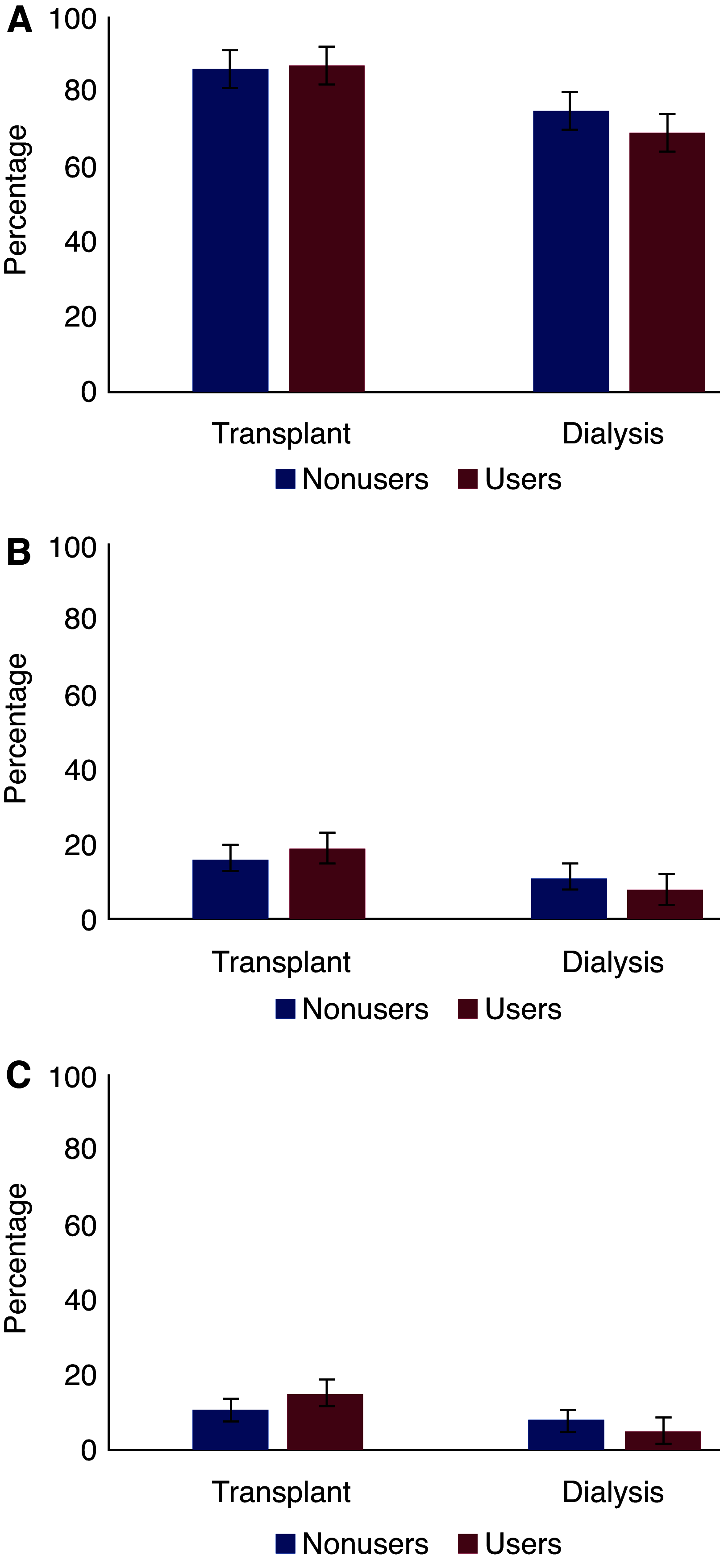
Results: We evaluated 1511 patients: 459 kidney transplant recipients and 1052 patients on dialysis. At diagnosis of COVID-19, 189 (41%) of the transplant recipients and 288 (27%) of the patients on dialysis were on ACEis/ARBs. A total of 88 (19%) transplant recipients and 244 (23%) patients on dialysis died within 28 days of initial presentation. In both groups of patients, there was no association between ACEi/ARB use and 28-day mortality in both crude and adjusted models (in transplant recipients, adjusted hazard ratio, 1.12; 95% confidence interval [95% CI], 0.69 to 1.83; in patients on dialysis, adjusted hazard ratio, 1.04; 95% CI, 0.73 to 1.47). Among transplant recipients, ACEi/ARB discontinuation was associated with a higher mortality risk after adjustment for demographics and comorbidities, but the association was no longer statistically significant after adjustment for severity of COVID-19 (adjusted hazard ratio, 1.36; 95% CI, 0.40 to 4.58). Among patients on dialysis, ACEi/ARB discontinuation was not associated with mortality in any model. We obtained similar results across subgroups when ACEis and ARBs were studied separately, and when other outcomes for severity of COVID-19 were studied, e.g., hospital admission, admission to the intensive care unit, or need for ventilator support.
Conclusions: Among kidney transplant recipients and patients on dialysis with COVID-19, there was no significant association of ACEi/ARB use or discontinuation with mortality.
Keywords: COVID-19; dialysis; kidney failure; kidney transplantation; renin angiotensin system.
Copyright © 2021 by the American Society of Nephrology.
Figures

References
-
- Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D: Localization of ACE2 in the renal vasculature: Amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol 296: F398–F405, 2009 - PubMed
-
- Anguiano L, Riera M, Pascual J, Valdivielso JM, Barrios C, Betriu A, Mojal S, Fernández E, Soler MJ; NEFRONA study: Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant 30: 1176–1185, 2015 - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL: A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

