Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release
- PMID: 34088740
- PMCID: PMC8183203
- DOI: 10.1136/jitc-2021-002488
Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release
Abstract
Background: Therapeutic options currently available for metastatic castration-resistant prostate cancer (mCRPC) do not extend median overall survival >6 months. Therefore, the development of novel and effective therapies for mCRPC represents an urgent medical need. T cell engagers (TCEs) have emerged as a promising approach for the treatment of mCRPC due to their targeted mechanism of action. However, challenges remain in the clinic due to the limited efficacy of TCEs observed thus far in solid tumors as well as the toxicities associated with cytokine release syndrome (CRS) due to the usage of high-affinity anti-CD3 moieties such as OKT3.
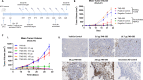
Methods: Using genetically engineered transgenic rats (UniRat and OmniFlic) that express fully human IgG antibodies together with an NGS-based antibody discovery pipeline, we developed TNB-585, an anti-CD3xPSMA TCE for the treatment of mCRPC. TNB-585 pairs a tumor-targeting anti-PSMA arm together with a unique, low-affinity anti-CD3 arm in bispecific format. We tested TNB-585 in T cell-redirected cytotoxicity assays against PSMA+ tumor cells in both two-dimensional (2D) cultures and three-dimensional (3D) spheroids as well as against patient-derived prostate tumor cells. Cytokines were measured in culture supernatants to assess the ability of TNB-585 to induce tumor killing with low cytokine release. TNB-585-mediated T cell activation, proliferation, and cytotoxic granule formation were measured to investigate the mechanism of action. Additionally, TNB-585 efficacy was evaluated in vivo against C4-2 tumor-bearing NCG mice.
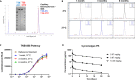
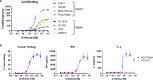
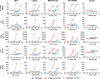
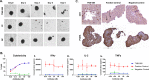
Results: In vitro, TNB-585 induced activation and proliferation of human T cells resulting in the killing of PSMA+ prostate tumor cells in both 2D cultures and 3D spheroids with minimal cytokine release and reduced regulatory T cell activation compared with a positive control antibody that contains the same anti-PSMA arm but a higher affinity anti-CD3 arm (comparable with OKT3). In addition, TNB-585 demonstrated potent efficacy against patient-derived prostate tumors ex vivo and induced immune cell infiltration and dose-dependent tumor regression in vivo.
Conclusions: Our data suggest that TNB-585, with its low-affinity anti-CD3, may be efficacious while inducing a lower incidence and severity of CRS in patients with prostate cancer compared with TCEs that incorporate high-affinity anti-CD3 domains.
Keywords: T-lymphocytes; cytokines; immunotherapy; prostatic neoplasms.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors except AS, SK, and LF were employees of Teneobio, Inc with equity interests when the reported work was conducted. LF and RD are consultants of Teneobio, Inc.
Figures


References
-
- National Cancer Institute . Seer cancer STAT facts: prostate cancer. Available: https://seer.cancer.gov/statfacts/html/prost.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
