Secondary Progressive Multiple Sclerosis: New Insights
- PMID: 34088878
- PMCID: PMC8397587
- DOI: 10.1212/WNL.0000000000012323
Secondary Progressive Multiple Sclerosis: New Insights
Abstract
In most cases, multiple sclerosis (MS) begins with a relapsing-remitting course followed by insidious disability worsening that is independent from clinically apparent relapses and is termed secondary progressive MS (SMPS). Major differences exist between relapsing-remitting MS (RRMS) and SPMS, especially regarding therapeutic response to treatment. This review provides an overview of the pathology, differentiation, and challenges in the diagnosis and treatment of SPMS. We emphasize the criticality of conversion from a relapsing-remitting to a secondary progressive disease course not only because such conversion is evidence of disability progression, but also because, until recently, treatments that effectively reduced disability progression in relapsing MS were not proven to be effective in SPMS. Clear clinical, imaging, immunologic, or pathologic criteria marking the transition from RRMS to SPMS have not yet been established. Early identification of SPMS will require tools that, together with the use of appropriate treatments, may result in better long-term outcomes for the population of patients with SPMS.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
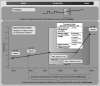
References
-
- Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647-656. - PubMed
-
- Kappos L, Bar-Or A, Cree BAC, et al. . Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. - PubMed
-
- Novartis Pharmaceuticals Corporation. Siponimod highlights of prescribing information. Accessed January 16, 2020, novartis.us/sites/www.novartis.us/files/mayzent.pdf.
-
- Novartis Pharma GmbH. Mayzent summary of product characteristics. Accessed February 5, 2020. ema.europa.eu/en/documents/product-information/mayzent-epar-product-info....
